The consensus to date is that most infants and children only have mild COVID‐19 symptoms and few require intensive care. 1 However, there are some described exceptions 2 , 3 , 4 and this brief report looks at a set of preterm twins born at the University Children's Hospital in Uppsala Sweden. The girl only had mild respiratory symptoms and stayed 24 hours at the paediatric ward and was then cared for at home, but the boy required intensive care and invasive ventilatory support.
The twins, of African descent, were delivered by Caesarean section at a postmenstrual age (PMA) of 30 weeks, as the mother had pre‐eclampsia and the male twin was showing intrauterine growth restriction. The boy was small for gestational age, with a birthweight of 1105 g. He was intubated and received surfactant due to infant respiratory distress syndrome and was then supported with continuous positive airway pressure therapy for another 24 hours. At PMA of 36 weeks, he had repeated severe apnoeas and needed resuscitation. Infection and oesophageal fistula were excluded, and polysomnographic registration showed an immature breathing pattern with central apnoeas. He was discharged at 38 weeks with caffeine citrate, continuous pulse oximetry and additional night‐time electrocardiography monitoring. During the first week at home, he had no apnoeas, desaturations or alarms on the monitor. However, the parents said they needed to carry and comfort him constantly, while his twin sister was more satisfied.
At 39 weeks, he developed severe apnoeas and was taken by ambulance to the hospital's emergency room. He presented with irritability, tachycardia and rhonchi. After broad microbiological sampling, he was prescribed two intravenous antibiotics: Cefotaxime (50 mg/kg three times daily) and Gentamicin (5 mg/kg daily). He was admitted to the paediatric ward for respiratory support with high‐flow nasal cannula oxygen therapy, and his body weight at admission was 2700 g. Within a few hours, reverse‐transcription polymerase chain reaction (RT‐PCR) for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) tested positive. The twin sister had mild symptoms and tested negative. Both parents were asymptomatic and tested positive. Three sisters, aged three, six and eight years, were asymptomatic and not tested.
The boy was transferred to the Pediatric Intensive Care Unit (PICU) due to frequent and severe apnoeas. He was orally intubated with a size 3.0 Microcuff endotracheal tube due to lethargy and ventilated in pressure control mode, with a Servo‐U Ventilator. The initial settings were a fraction of inspired oxygen (FiO2) of 0.3, positive end‐expiratory pressure (PEEP) of 5 cm H2O, pressure control of 16 cm H2O, respiratory rate of 45 per minute and an I:E ratio of 1:2. The resultant tidal volume was 5.3 mL/kg, with a mean airway pressure of 10.2 cm H2O and oxygen saturation of 98%.
The infant remained on the ventilator for the next 6 days. Automode pressure control/pressure support was used, with a PEEP level of 6‐10 cm H2O, peak pressure maintained below 28 cm H2O, mean airway pressure of 5.4‐12.6 cm H2O and a FiO2 of 0.25‐0.45 for a target saturation above 92% and normocapnia. Tidal volumes varied between 4 and 7 mL/kg. Gentle recruitment manoeuvres were carried out as necessary, with incremental PEEP of up to 10 cm H2O (Figure 1).
FIGURE 1.
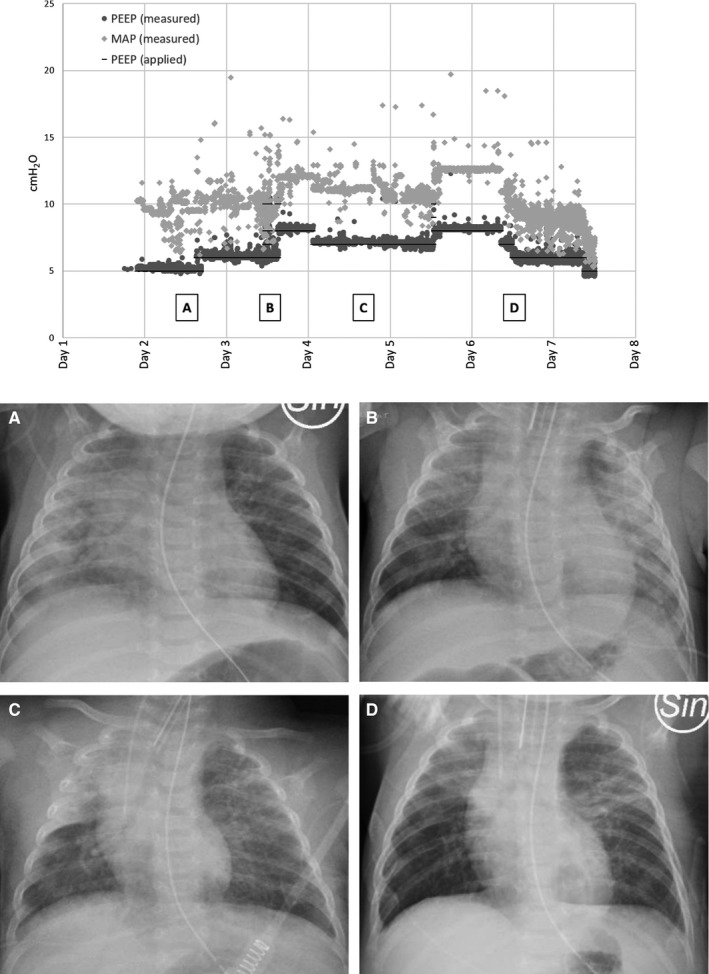
Applied and measured PEEP and mean airway pressure in cm H2O versus ventilatory support days. A, B, C and D denote when the chest X‐ray images were obtained
The prone position was periodically used during the first 3 days in the PICU, and the oxygenation index decreased from 9.57 to a normal level of 3.39.
The airway secretions were initially very thick. Closed endotracheal suctioning and active humidification system were applied. Inhalation with isotonic saline started directly after intubation and was given hourly. Acetylcysteine inhalation was added from the second to third PICU day, with good effects on secretion removal. Due to previous apnoeas, caffeine citrate (10 mg/kg daily) was continued during his stay.
Chest X‐rays showed variable bilateral consolidations, initially diffuse infiltrates predominantly in the right lung. (Figure 1). The laboratory findings showed no signs of exuberant inflammatory response. The abnormalities that were found were mild increases in procalcitonin, interleukin‐6, D‐dimer, ferritin and mild pancytopenia, including lymphocytopenia. The patient's serum creatinine concentration, aspartate aminotransferase, alanine aminotransferase, N‐terminal prohormone of brain natriuretic peptide and triglycerides were within the normal ranges.
Nasopharyngeal and oropharyngeal swabs were collected, and SARS‐CoV‐2 was detected with RT‐PCR. Repeated tests were still positive on day eight. The virus was also detected in tracheal and faeces specimens.
The infant was normothermic during his entire hospital stay. Antibiotics were discontinued when tracheal cultures showed negative result. Blood cultures and tracheal polymerase chain reaction (PCR) for Mycoplasma pneumoniae and Chlamydia pneumoniae were negative. Assays for influenza viruses and a respiratory panel were all negative. Screening laboratory tests for immunodeficiency were unremarkable. A lumbar puncture was performed on day three because of irritability. The cerebrospinal fluid culture was negative, the cytology was normal and PCR was negative for SARS‐CoV‐2 and herpes simplex virus 1 and 2.
Enteral nutrition was given with maternal breast milk, possibly supporting the immune response with immunoglobulin A and other immunomodulating factors. The infant was sedated with infusions of morphine and midazolam. Thrombosis prophylaxis was considered, due to reports on thromboembolic complications in adults with COVID‐19, but deemed unfavourable.
Gas exchange improved on day six, and sedation was tapered. The next day, he was extubated and managed with high‐flow nasal cannula with low FiO2 that was stopped after a couple of hours. No significant apnoeas were registered. After 2 days of uneventful observation, the patient was discharged home.
The take home message is that even infants can get severe COVID‐19 that may require intensive care and invasive ventilatory support. Possible risk factors in this particular case may have been high and repeated viral exposure, preterm birth, immature regulation of breathing, African descent and male gender.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
We want to thank our Uppsala colleagues for their help and advice: Associate Professor Christian Sundberg and Deputy Chief Pharmacist Mattias Paulsson PhD, Department of Women's and Children's Health, and Professor Miklós Lipcsey, Department of Surgical Sciences.
REFERENCES
- 1. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.0878. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui Y, Tian M, Huang D, et al. A 55‐day‐old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronado Munoz A, Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late‐onset neonatal sepsis in a patient with Covid‐19. N Engl J Med. 2020;382(19):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]


