A 24‐year‐old man with Crohn’s disease (CD) was admitted to our hospital with a 9‐day history of skin rash, severe asymmetric arthralgia, periarticular swelling, and abdominal pain. He had no respiratory symptoms or fever. He had been diagnosed as having CD in 2016 and underwent ileocecal resection in 2017. He had been receiving adalimumab since surgery and his disease was considered to be in remission. Despite the moderately severe, diffuse abdominal pain, he had no intestinal bleeding or diarrhea. Physical examination revealed palpable purpura on the legs and arms, swelling of the left hand, and pain on palpation of several joints without signs of arthritis. He was afebrile, and pulse and blood pressure were normal.
Nasopharyngeal swab for coronavirus disease 2019 (COVID‐19) was performed at admission and was found to be positive using polymerase chain reaction (PCR) (Xpert Xpress SARS–CoV‐2). Blood cell count, urinalysis results, liver test results, and creatinine and lipase levels were normal. Elevated levels of C‐reactive protein (44 mg/liter), d‐dimer (5,470 ng/ml), fibrinogen (4.6 gm/liter), and complement C4 (0.48 gm/liter) were found. No other viral agents were identified.
Serum IgA levels were markedly increased (5.3 gm/liter), while levels of IgG and IgM were normal. Serologic testing for COVID‐19 (Euroimmun) revealed IgA but no IgG, and upon retesting 10 days later, the patient was negative for both IgA and IgG. Although nasopharyngeal swab results were positive for COVID‐19 at admission, they were negative on 2 consecutive days after admission, and results of a PCR study for severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2) in the stool were negative.
Computed tomography (CT) showed extended ileitis with marked circumferential bowel wall thickening and hyperenhancement of the inner mucosa and submucosal edema, a feature typical of vasculitis involvement. CT of the chest showed no abnormalities.
Skin biopsy confirmed a diagnosis of IgA vasculitis, with perivascular and vessel wall infiltration by neutrophils and lymphocytes, leukocytoclasia, and C3 and IgA deposits in dermal capillaries identified using immunofluorescence staining (Figure 1).
Figure 1.
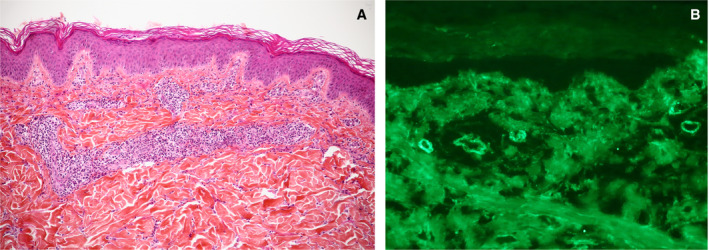
Skin biopsy showing superficial dermis small vessel vasculitis with lymphocytes, neutrophils, and leukocytoclasia (A), with dermal capillary surface vascular deposits of IgA (B) and C3, but not IgG, revealed by direct cutaneous immunofluorescence staining. Original magnification × 10 in A, × 20 in B.
Low molecular weight heparin and intravenous steroids (methylprednisolone 0.8 mg/kg) were prescribed 2 days after admission for intense abdominal pain. The patient was discharged on day 7, receiving oral steroids and enoxaparin.
IgA vasculitis is a systemic small vessel vasculitis that may be triggered by different microorganisms (1). This case of IGA vasculitis in a CD patient receiving anti–tumor necrosis factor therapy, as reported (2), is remarkable for several reasons. First, it was associated with COVID‐19, confirmed by PCR and serologic testing. The patient was admitted during the peak of the COVID‐19 pandemic and had come into contact with several people who were potentially COVID‐19 positive but untested. Laboratory test results were remarkable for high levels of d‐dimer and inflammation markers, suggesting a hypercoagulable state, which is one of the notable characteristics of COVID‐19. A second notable finding was high IgA levels in the serum, with weak and transitory positivity only for IgA on COVID‐19 serologic testing. As previously reported (3), anti–SARS–CoV‐2 IgA is the first immunoglobulin detectable after COVID‐19 infection. There is some evidence of other IgA‐related diseases being associated with COVID‐19. Indeed, one report has suggested a possible association between COVID‐19 and Kawasaki disease (4), a systemic vasculitis associated with an antigen‐driven IgA response (5). Interestingly, increased intestinal permeability has been reported in this disease, suggesting that disrupted intestinal barrier function plays a role in the development of IgA vasculitis (6). Furthermore, chilblain‐like lesions with possible vascular damage have been reported to be possibly linked to COVID‐19 infection, with anti–COVID‐19 serologic testing revealing IgA but no IgG in several patients (7).
Even if we cannot prove the causality of COVID‐19, it is notable that in this patient, IgA vasculitis was associated with elevated levels of serum IgA and with only IgA shown on COVID‐19 serologic testing. Endothelial injury during COVID‐19 infection has recently been reported, with a recent study suggesting that SARS–CoV‐2 infection participates in the induction of endotheliitis in several organs as a direct consequence of viral involvement and the host inflammatory response (8).
Dr. Allez has received consulting fees, speaking fees, and/or honoraria from Amgen, Biogen, Boehringer Ingelheim, Celgene, Ferring, Genentech, Janssen, Pfizer, Takeda, Tillotts, and Roche (less than $10,000 each) and research support from Janssen and Genentech. Dr. Battistella has received consulting fees, speaking fees, and/or honoraria from Bristol Myers Squibb, Innate Pharma, and Kyowa Kirin (less than $10,000 each) and research support from Takeda. Dr. Molina has received consulting fees and/or honoraria from Gilead, Merck, ViiV, and Sanofi (less than $10,000 each) and research support from Gilead. No other disclosures relevant to this letter were reported.
References
- 1. Audemard‐Verger A, Pillebout E, Guillevin L, Thervet E, Terrier B. IgA vasculitis (Henoch‐Shönlein purpura) in adults: diagnostic and therapeutic aspects [review]. Autoimmun Rev 2015;14:579–85. [DOI] [PubMed] [Google Scholar]
- 2. Marques I, Lagos A, Reis J, Pinto A, Neves B. Reversible Henoch‐Schönlein purpura complicating adalimumab therapy. J Crohns Colitis 2012;6:796–9. [DOI] [PubMed] [Google Scholar]
- 3. Padoan A, Sciacovelli L, Basso D, Negrini D, Zuin S, Cosma C, et al. IgA‐Ab response to spike glycoprotein of SARS‐CoV-2 in patients with COVID‐19: a longitudinal study. Clin Chim Acta 2020;507:164–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viner RM, Whittaker E. Kawasaki‐like disease: emerging complication during the COVID‐19 pandemic. Lancet 2020;395:1741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rowley AH, Shulman ST, Garcia FL, Guzman‐Cottrill JA, Miura M, Lee HL, et al. Cloning the arterial IgA antibody response during acute Kawasaki disease. J Immunol 2005;175:8386–91. [DOI] [PubMed] [Google Scholar]
- 6. Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, et al. Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity 2019;51:508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Hachem M, Diociaiuti A, Concato C, Carsetti R, Carnevale C, Ciofi Degli Atti M, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


