It is almost 6 months since novel coronavirus disease 2019 (COVID‐19) started as a local outbreak in Wuhan, China 1 For hematologists and patients suffering from hematological disorders, the COVID‐19 pandemic has brought challenges with regard to the diagnosis, treatment, follow‐up, office visits, and so on. 2 , 3 We hereby discuss the current challenges for management of immune thrombocytopenia (ITP) during COVID‐19 pandemic, based on our practical experience and interim expert recommendations suggested by the hematology societies.
1. THROMBOCYTOPENIA IN CORONAVIRUS DISEASE 2019
Thrombocytopenia, in general, is one of the very commonly encountered laboratory anomalies in hematological practice. It is a challenging entity because of multiple reasons which can interplay to cause thrombocytopenia. Thrombocytopenia, and lymphopenia, has been found as common hematological abnormalities in patients with COVID‐19, which has also been shown to be an indicator of poor outcome and survival. 4 , 5 The prevalence of thrombocytopenia is extremely variable. Huang et al noted COVID‐19 non‐survivors (20%) to be more thrombocytopenic than survivors (1%). 6 In most COVID‐19 cases of thrombocytopenia, the platelet count does not fall below 100 × 109/L. Severe degree of thrombocytopenia (<20 × 109/L, or a sudden drop >50% over 24‐48 hours) narrows down the differentials to ITP, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, microangiopathic hemolytic anemia, heparin induced thrombocytopenia, or disseminated intravascular coagulation. 7 , 8
But before considering the diagnosis of ITP, it is important to evaluate patients with other treatable causes of thrombocytopenia. 7 , 9 The approach to ITP is based on the clinical history, systemic examination, and a detailed hematological work up including a complete blood count, and peripheral smear examination. It is recommended to test patients for HCV and HIV to rule out this viral mediated thrombocytopenia. Guidelines do not recommend bone marrow examination in cases with isolated thrombocytopenia unless there is a concern for aplastic anemia. leukemia, or myelodysplastic syndrome. 10 Other immune conditions like antiphospholipid syndrome (APLA) could present with thrombocytopenia. 11 Revised classification criteria for the APLA with clinical and lab criteria in making the diagnosis of APLA in such scenarios.
Based on the above‐mentioned points, three scenarios could be expected for any hospitalized patient with COVID‐19 and thrombocytopenia, (Figure 1):
-
(1)
Known ITP (chronic), who develops COVID‐19.
-
(2)
New onset ITP (acute) and COVID‐19 at the same time and for the first time.
-
(3)
Thrombocytopenia in patients with COVID‐19 unrelated to ITP.
Figure 1.

Descriptive figure suggesting the broader categories of scenarios possible in a patient with coronavirus disease 2019 (COVID‐19). ITP, immune thrombocytopenia
For evaluation of all these three categories, a review of previous records is crucial, as it might save a lot of time and prevent from ordering unnecessary investigation for the evaluation of thrombocytopenia while taking care of a patient with COVID‐19. Also, it is important to note that the timing of thrombocytopenia in patients with COVID‐19 can be remarkably variable and patients with COVID‐19 can rarely present in the form of acute ITP or relapse. 9
While approaching a suspected case of ITP, it is important to look for other common causes of thrombocytopenia and determining if ITP is primary versus secondary.
As the universal bearing of COVID‐19 grows daily, the International ITP Coalition remains devoted to the health and safety of the patients with ITP. The Platelet Disorder Support Association, American Society of Hematology (ASH), British Society of Hematology (BSH) and other international hematology societies are working relentlessly to ensure that constant updates and developments made readily available in the form of interim guidance to the treating physicians and hematologists worldwide 12 , 14
2. ITP IN COVID‐19
Like any viral illness, severe acute respiratory syndrome coronavirus 2 could potentially trigger a new event of ITP. Recently, Zulfiqar et al reported a case of newly diagnosed ITP in a 65‐year‐old female with COVID‐19 that required a sequential trial of multiple drugs, including intravenous immunoglobulin (IVIG), prednisolone, and eltrombopag for complete recovery. 15
The consensus is that if required, non‐immunosuppressant treatment modalities should be considered above the immunosuppressant treatment strategies, while managing ITP during the COVID‐19 pandemic. The commonly used non‐immunosuppressant is IVIG and TPO analogs like romiplostim and eltrombopag. Both ASH and BSH have given the interim recommendations for managing patients with ITP in COVID‐19 (Table 1). BSH precisely mentions about the differential approach to acute ITP in COVID‐19 positive and COVID‐19 negative patients. BSH recommends considering steroids over thrombopoietin receptor agonists (TPO‐RAs) in COVID‐19 positive cases and using TPO‐RAs (off label) as the first line in COVID‐19 negative patients. COVID‐19 positive patients are at increased risk of the thrombo‐embolic phenomenon and hence steroids are preferred over TPO agents. Use of steroids and TPO‐RAs is based on the risk of increased mortality and superadded infection associated with steroids (in high doses) versus increased thrombotic potential while using TPO‐RAs. 6
Table 1.
Various scenarios of ITP with COVID‐19 and their management
| Scenario | Recommendations | Society |
|---|---|---|
| New onset acute or relapsed chronic ITP COVID‐19 positive. | Consider steroids >TPO‐RAs (because of concerns of increased thrombosis with TPO‐RAs) non‐bleeding patients
|
BSH |
| New onset acute or relapsed chronic ITP without COVID‐19 symptoms or COVID‐19 negative test | TPO‐RAs may be preferred as first line treatment >steroids(because of the concerns of the steroids causing immunosuppression and predisposition to COVID‐19) caution:
|
BSH |
| Chronic ITP patients already on TPO‐RAs (hospitalized for COVID‐19) | ‐Trial of dose increment of TPO agent or a addition of second one (eg, add romiplostim to eltrombopag or avatrombopag or add eltrombopag or avatrombopag to romiplostim) | ASH |
| Chronic stable ITP without COVID‐19 symptoms |
|
|
| Splenectomised patients |
|
(Both ASH and BSH) |
| Thromboprophylaxis in patients with ITP hospitalized for COVID‐19 |
|
BSH |
Note: Synopsis of BSH and ASH recommendations.
Abbreviations: ASH, American Society of Hematology; BSH, British Society of Hematology; COVID‐19, coronavirus disease 2019; DIC, disseminated intravascular coagulation; ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; LMWH, low‐molecular‐weight heparin; TPO, thrombopoietin; TPO‐RA, thrombopoietin receptor agonist.
We believe that every patient with ITP brings his or her own challenge and due to lack of enough evidence, management should be based on the combination of available data, additional experience, and interim recommendations given by hematology societies (figure 2).
Figure 2.
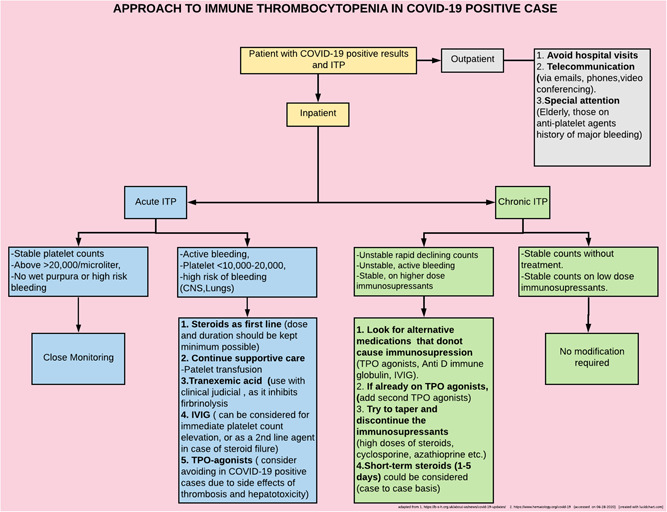
Proposed flowchart to approach ITP patients with COVID‐19 (based on interim recommendations by American society of hematology and British society of hematology). CNS, central nervous system; COVID‐19, coronavirus disease 2019; ITP, immune thrombocytopenia; TPO, thrombopoietin
3. NEW ACUTE/RELAPSED CHRONIC ITP WITH POSITIVE COVID‐19
TPO‐RAs are associated with increased thrombotic potential, hence there is always a risk of promoting thromboembolic episodes in patients with COVID‐19. Recent recommendation published in the British Journal of Hematology hence proposed steroids as a preferred first‐line therapy than TPO‐RAs. Steroids are not risk‐free and there are concerns of increased mortality and secondary infection at higher doses (>40 mg methylprednisolone/day). Hence, the BSH recommended that steroid dose and duration should be kept to the minimum with an initial dose of 20 mg daily followed by a tapering trial after 2 weeks. Both BSH and ASH recommend using IVIG as a bridging therapy for dangerously low platelet counts of less than 10 to 30 × 109/L, while waiting for the response of steroids or TPO‐RAs. The use of platelet transfusion should be restricted only for major bleeding.
Unlike BSH that expressed its concerns over thrombotic side‐effects of TPO agents, ASH suggests that those chronic ITP patients with newly identified COVID‐19, who are already on TPO agents in the case of relapse to either increase the dose or to add a second TPO agent. 9
4. NEW ACUTE/RELAPSED CHRONIC ITP WITHOUT COVID‐19 SYMPTOMS OR NEGATIVE COVID‐19 TEST
This category includes patients who have newly detected or relapsed chronic ITP without any COVID‐19 related concerns or were found to be COVID‐19 negative upon testing. BSH recommends TPO‐RAs (off label) as the first line over steroids. The idea of not using steroids in COVID‐19 negative patients is to keep their immune system active against acquiring COVID‐19. 7 The challenge of TPO‐RAs would be a delayed onset of action (10‐14 days) that might require using IVIG or platelet transfusions as needed.
5. CHRONIC ITP WITH STABLE COUNTS WITHOUT COVID‐19 SYMPTOMS
Chronic ITP patients with stable platelet counts should be extremely cautious during the COVID‐19 pandemic. BSH recommends continuing steroids and immunosuppressants in general, while ASH recommendation varies based on whether the patient is on low dose versus high dose of immunosuppressants. 12 , 13 ASH recommends smaller doses of steroids/immunosuppressants need not be changed, but recommends to consider tapering and possibly discontinuation of high doses by using alternative medications like TPO‐RAs and/or IVIG.
6. CLOSING THE KNOWLEDGE GAP OF PATIENTS WITH ITP DURING COVID‐19 PANDEMIC
Patients with ITP may have some concerns about their risk to acquire COVID‐19 or should they change or adjust ITP medications? It is particularly important to address these concerns because the COVID‐19 pandemic is likely to sustain for at least a few months, if not years. British Society for Rheumatology suggested following patients to be more vulnerable as compared with the others to COVID‐19.
Patients on corticosteroids ≥20 mg/day (0.5 mg/kg), prednisolone (or equivalent) for more than 4 weeks.
Patients on corticosteroid dose of ≥5 mg/day prednisolone (or equivalent) for more than 4 weeks AND at least one other immunosuppressive medication or rituximab within the last 1 year. 16
Patients taking a combination of two immunosuppressants, including rituximab within the last 12 months AND an additional co‐morbidity.
Patients should be encouraged to utilize the resources like telemedicine to reach out to their respective ITP clinics for immediate questions regarding their disease. Every attempt should be made to avoid unnecessary hospital visits and educating patients with proper resources could be a key factor to reduce their anxiety and motivate them to keep following all the hygiene practices and social distancing (Figure 3).
Figure 3.

Description of patient information (based on BSH/ASH) regarding ITP in COVID‐19. ASH, American Society of Hematology; BSH, British Society of Hematology; COVID‐19, coronavirus disease 2019; ITP, immune thrombocytopenia; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TPO, thrombopoietin
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
All authors have seen the manuscript and agree to the content and data. All the authors played a significant role in the paper.
Sahu KK, Siddiqui AD, Rezaei N, Cerny J. Challenges for management of immune thrombocytopenia during COVID‐19 pandemic. J Med Virol. 2020;92:2277–2282. 10.1002/jmv.26251
REFERENCES
- 1. Sahu KK, Lal A, Mishra AK. Latest updates on COVID‐2019: a changing paradigm shift. J Med Virol. 2020;92:533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahu KK, Siddiqui AD. From hematologist's desk: the effect of COVID‐19 on the blood system. Am J Hematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahu KK, Siddiqui AD, Cerny J. Managing sickle cell patients with COVID‐19 infection: the need to pool our collective experience. British J Haematol. 2020. https://onlinelibrary.wiley.com/doi/abs/10.1111/bjh.16880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra AK, Sahu KK, George AA, Lal A. A review of cardiac manifestations and predictors of outcome in patients with COVID‐19. Heart Lung. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. 2020;382(18):1708‐1720. https://www.nejm.org/doi/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhibar DP, Sahu KK, Dhir V, Singh S. Immune thrombocytopenia as a presenting manifestation of tuberculosis‐ challenge in resource constraint settings. J Clin Diagn Res. 2016;10(10):OD01‐OD02. https://pubmed.ncbi.nlm.nih.gov/27891377/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahu KK, Varma SC. Cortical vein thrombosis in a case of idiopathic thrombocytopenic purpura. Platelets. 2015;26:374‐375. [DOI] [PubMed] [Google Scholar]
- 9. Murt A A, Eskazan AE, Yılmaz U, Ozkan T, Ar MC. COVID‐19 presenting with immune thrombocytopenia: a case report and review of the literature. J Med Virol. 2020. https://pubmed.ncbi.nlm.nih.gov/32497344/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4207. https://pubmed.ncbi.nlm.nih.gov/21325604/ [DOI] [PubMed] [Google Scholar]
- 11. Thrombocytopenia in the antiphospholipid syndrome: pathophysiology, clinical relevance and treatment. 2020. https://pubmed.ncbi.nlm.nih.gov/8952756/ [PubMed]
- 12. COVID‐19. British Society for Haematology. 2020. https://b-s-h.org.uk/about-us/news/covid-19-updates/
- 13.COVID‐19 and ITP—Hematology.org. 2020. https://www.hematology.org/covid-19/covid-19-and-itp
- 14. Platelet Disorder Support Association—for People with ITP—Home. 2020. https://www.pdsa.org/
- 15. Zulfiqar AA, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with covid‐19. New Eng J Med. 2020;382:E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. COVID‐19. 2020. https://www.rheumatology.org.uk/covid-19/


