Editor
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2), was first reported in China on December 2019. Almost 5 months into the pandemic, little is still known about cutaneous manifestations in COVID‐19. In fact, the prevalence of cutaneous signs varies greatly in the literature, ranging from 0.2% to 20.4%. 1 , 2
Given their potential association with COVID‐19, acral lesions have received special attention worldwide, both in the medical literature and the media. Our aim is to share our experience regarding acral manifestations during this pandemic.
We report a case series including all patients consulting at our tertiary care dermatology department for suspected COVID‐associated cutaneous lesions during April 2020, in particular, those with acral lesions. All patients were tested for SARS‐CoV‐2 through nasopharyngeal exudate polymerase chain reaction (PCR) and serum serology, as well as for other exanthematic viruses [parvovirus B‐19, measles, rubella and human herpesvirus‐6 (HHV6)].
Twenty‐six patients were included, 14 (54%) men and 12 (46%) women. The mean age was 28 years, noting 8 (30.7%) were under the age of 14. Most cases were urgent outpatient consultations. The predominant manifestation was acral rash (19/26; 73.08%). Less common eruptions were maculopapular (4/26; 15.38%), urticariform (2/26; 7.69%) and chickenpox‐like rashes (1/26; 3.85%).
Regarding the 19 patients with acral manifestations, they presented perniosis‐like lesions with variable degrees of erythema, oedema and petechiae or purpuric macules. Thirteen (68.42%) referred pruritus and 9 (47.36%) pain. None had respiratory symptoms, and only 2 (10.5%) reported having fever previously. We found that nasopharyngeal PCR was negative in all cases at onset of lesions. Most patients tested negative for SARS‐CoV‐2 serology and presented with predominantly petechial or purpuric lesions (Fig. 1). Only 3 (15.78%) cases had positive serology: two with IgG and one with both IgG and IgA, suggesting recent infection. The former two presented purpuric lesions, whereas the latter had erythematosquamous lesions on the fingers, elbows and knees (Fig. 2). Two patients had been previously confirmed to have COVID through PCR, approximately 15 days earlier. These included 1 patient with acral purpuric lesions and positive IgG, and the patient with erythematosquamous lesions and both IgG and IgA. Two patients with purpuric lesions tested positive only for other viruses: HHV‐6 nasopharyngeal PCR and parvovirus B‐19 serum PCR, of unknown significance.
Figure 1.

Acral lesions in patients with negative nasopharyngeal PCR and serology for COVID‐19: (a) Petechial and purpuric macules on the dorsal aspect of the toes in a 19‐year‐old male. (b) Erythematoedematous lesions with purpuric hue and digital swelling on the distal aspect of the toes in a 13‐year‐old female. (c, d) Purpuric macules with haemorrhagic vesicle on the fingers of a 41‐year‐old male. (e) Erythema and oedema on multiple toes in a 19‐year‐old male.
Figure 2.
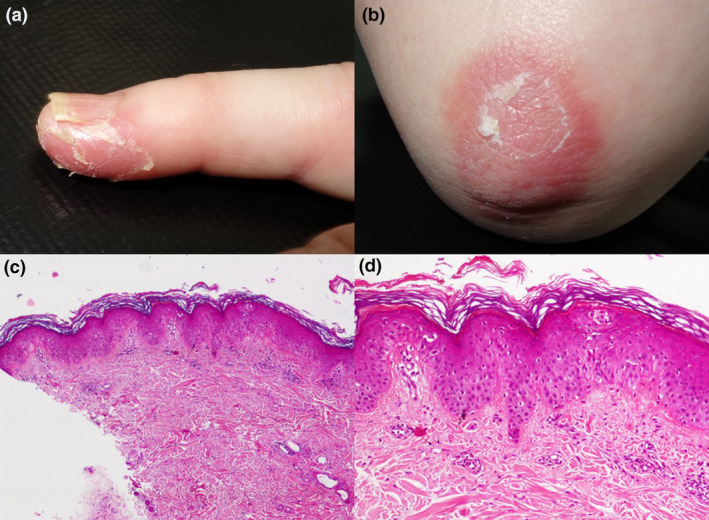
Clinical and histological findings of a 63‐year‐old female with perniosis‐like lesions and positive SARS‐CoV‐2 serum IgG and IgA. (a) Erythema and desquamation on the distal portion of the fifth finger. (b) Erythematosquamous plaque on the elbow. (c, d) Skin biopsy from the elbow showing a superficial and perivascular lymphocytic infiltrate, dermal oedema with mild spongiosis and focal exocytosis (haematoxylin and eosin stain, ×40 and ×100).
Out of seven patients with non‐acral manifestations, 4 (57.14%) and 5 (71.42%) reported fever and respiratory symptoms, respectively, prior to cutaneous lesions. Nasopharyngeal PCR was negative in all cases at the moment of consultation. We identified two cases with positive IgG who presented with maculopapular and chickenpox‐like eruptions.
The ‘COVID‐piel’ study reported five rashes associated with COVID‐19, maculopapules and acral pseudo‐chilblain being the most frequent. 3 Recalti et al. reported two distinct acral rashes in association with COVID‐19: petechial acral eruption and digitate papulosquamous rash. These findings resemble those in our series. The timing of rash onset is strikingly variable, suggesting that skin findings may present at any stage of the infection. 4 Given that the cases we could relate to SARS‐CoV‐2 only had positive serology, perhaps acral lesions are a late COVID manifestation in patients with mild or no systemic symptoms.
Although we observed an increased number of consultations for acral lesions when compared with previous years, and coinciding with the peak incidence of COVID‐19 in our city, we could not demonstrate a relationship with SARS‐CoV‐2 in most cases. It is worth noting that our only patient with positive IgA and previous PCR presented different cutaneous manifestations, with erythematosquamous lesions. Perhaps this distinction could be useful when evaluating acral manifestations in the COVID‐19 era. Despite our efforts, there are still many unknowns regarding COVID‐related dermatological manifestations.
Conflicts of interest
Dr. García‐Legaz Martínez, Dr. Martínez‐Doménech, Dr. Magdaleno‐Tapial, Dr. Valenzuela‐Oñate, Dr. Partarrieu‐Mejías, Dr. Lorca‐Spröhnle, Dr. Casanova‐Esquembre, Dr. Pérez‐Ferriols and Dr. Alegre‐de Miquel have nothing to disclose.
Funding source
None.
References
- 1. Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID‐19 global pandemic. JAMA Dermatol 2020. Published online. [DOI] [PubMed] [Google Scholar]
- 2. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 3. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐ 19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020;183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgements
Concepción Gimeno‐Cardona, PhD (Department of Microbiology, Hospital General Universitario de Valencia, Valencia, Spain), David Navalpotro‐Rodríguez MD (Department of Microbiology, Hospital General Universitario de Valencia, Valencia, Spain), Eduardo Bernia‐Petit, MD (Department of Dermatology, Instituto Valenciano de Oncología, Valencia, Spain), Elisa Ríos‐Viñuela, MD (Department of Dermatology, Instituto Valenciano de Oncología, Valencia, Spain). The patients in this manuscript have given written informed consent to the publication of their case details.


