The use of high‐dose, high‐volume nasal steroid irrigation (HVNSI) has been incorporated into the medical management of recalcitrant chronic rhinosinusitis (CRS) after surgical intervention, and has been shown to be efficacious. 1 However, data are limited on the effectiveness of HVNSI in patients before surgical intervention. In a pilot study, we retrospectively evaluated a group of patients who were considered candidates for endoscopic sinus surgery (ESS). Instead of ESS, however, we pursued HVSI as an alternative therapy. This option has become particularly valuable in the era of coronavirus‐2019 (COVID‐19), where outpatient clinical and operative volume has been reduced in the interest of protecting patients, staff, and surgeons.
Current medical management of CRS with polyps (CRSwNP) and without polyps (CRSsNP) includes topical saline irrigations and steroid sprays with periodic systemic steroids for acute exacerbations. 1 Recurrent doses of systemic steroids may have a worse impact on the quality of life (QOL) than the CRS itself, 2 and systemic steroid use should be minimized. 3 Ideally, symptoms should be managed medically without such side effects and, if possible, ESS should be avoided or number of surgeries minimized. 4
Saline irrigations, with or without the off‐label addition of high‐dose topical steroids, have been shown to improve symptoms in patients with CRS as either the main modality or in combination with other treatments. 1 , 5 Currently, the impact of HVNSI on CRS patients is unknown with regard to limiting or delaying the need for surgical intervention. Traditionally, surgery is performed before initiation of HVNSI to maximize sinus access of topical therapy. At our institution, where HVNSI is frequently prescribed for CRS and utilized before surgical intervention, we sought to identify the value of HVNSI in patients who were candidates for ESS.
A pilot study was approved by the institutional review board of the University of Pennsylvania to evaluate patients with CRS cared for by one attending rhinologist over a 9‐year period. This was admittedly a highly heterogeneous population with regard to disease type, severity, and other treatment modalities, but it included patients with a diagnosis of CRSsNP or CRSwNP who completed 6 weeks of twice‐daily HVNSI (either mometasone or budesonide).
Overall, patients found this therapy to be acceptable and they were willing to trial HVNSI in an effort to avoid ESS. Ninety patients underwent HVNSI with budesonide or mometasone. The group was heterogeneous with regard to CRS phenotype, surgical history, medical comorbidities, and social history (Table 1). The majority of patients (64%) did not undergo ESS, and notably this did not differ based on CRS phenotype, surgical history, medical comorbidities, and social history. Scores on the 22‐item Sino‐Nasal Outcome Test (SNOT‐22) improved significantly after HVNSI in all patients, regardless of whether or not they went on to have ESS (p < 0.0001) (Fig. 1). Lund‐Mackay score improved after HVNSI, but this was only statistically significant in the patients who ultimately proceeded to ESS (p = 0.0102).
TABLE 1.
Baseline characteristics of patients (N = 90)
| Characteristics | |
|---|---|
| Gender | Males: N = 50 (55.6%); females: N = 40 (44.4%) |
| Smoker | Yes: N = 7 (7.8%); no: N = 83 (92.2%) |
| Mean age | 50.0 ± 17.89 years |
| Asthma | Yes: N = 31 (34.4%); no: N = 59 (65.6%) |
| Steroid used | Mometasone: N = 25 (27.8%); budesonide: N = 65 (72.2%) |
| CRS type | CRSwNP: N = 29 (32.2%); CRSsNP: N = 61 (67.8%) |
| Previous ESS | Yes: N = 30 (33.3%); no: N = 60 (66.7%) |
CRSsNP = chronic rhinosinusitus without nasal polyps; CRSwNP = chronic rhinosinusitus with nasal polyps; ESS = endoscopic sinus surgery.
FIGURE 1.
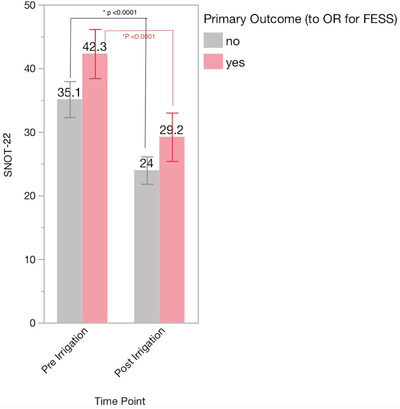
SNOT‐22 scores pre‐ vs post‐irrigation and the decision for ESS. ESS = endoscopic sinus surgery; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
Although this pilot study represents a limited evaluation from which we must temper our general conclusions, the principle is valuable. This is particularly true in the setting of the COVID‐19 pandemic, during which outpatient clinical and surgical volume has been significantly reduced, as we must prioritize nonsurgical therapies for patients who may otherwise qualify for ESS. In previous studies, there was a trend in support of early, upfront ESS to save patients from prolonged poor QOL, financial burden, and progression of symptoms, particularly asthmatic patients. 6 However, the findings demonstrate that patients with CRS who committed to ≥6 weeks of HVNSI had improved QOL outcome measures. In a majority of these patients, the improvement was significant enough to exclude them from ESS candidacy.
Further analysis showed that the probability of proceeding to ESS did not differ between CRS phenotypes, highlighting the fact that patients with both CRSwNP and CRSsNP can benefit from HVNSI. Importantly, the likelihood of proceeding to ESS did not differ between patients with and without a history of ESS, suggesting that patients may benefit from further medical management with HVNSI rather than upfront or early ESS if a patient is naive to this regimen. Although not approved by the US Food and Drug Administration (FDA), use of HVNSI has been investigated and, overall, appears safe for long‐term use, and this should be conveyed to patients. 7 , 8 Because of the lack of FDA approval, insurance coverage can be a limiting factor with regard to HVNSI accessibility. We found that use of direct mail ordering from the pharmacy helps limit patients’ costs.
The findings are limited given the nature of our pilot study, but some CRS patients have demonstrated significant improvements in pre‐ vs post‐irrigation SNOT‐22 scores. Our overall rate of patients proceeding to ESS despite an adequate trial of HVNSI was only 34%. This generally supports the use of HVNSI in patients with CRS, regardless of polyp status and surgical history. Future studies should provide prospective data aimed at matching patients based on age, sex, medical comorbidities, insurance status, and surgical history. In addition, comparing preoperative QOL measures in the context of HVNSI is necessary, as this is known to impact the decision for surgery. This is particularly important at a time when surgical intervention carries significant risks to the patient, staff, and surgeon, due to the novel coronavirus—a risk that will not fully resolve even after the surge has peaked.
Funding source for the study: Department of Otorhinolaryngology, University of Pennsylvania.
Potential conflict of interest: None provided.
View this article online at wileyonlinelibrary.com.
References
- 1. Harvey R, Snidvongs K, Kalish LH, Oakley GM, Sacks R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double‐blinded placebo‐controlled trial for chronic rhinosinusitis after sinus surgery. Int Forum Allergy Rhinol. 2018;8:461‐470. [DOI] [PubMed] [Google Scholar]
- 2. Leung RM, Dinnie K, Smith TL. When do the risks of repeated courses of corticosteroids exceed the risks of surgery? Int Forum Allergy Rhinol. 2014;4:871‐876. [DOI] [PubMed] [Google Scholar]
- 3. Head K, Chong LY, Hopkins C, Philpott C, Schilder AG, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016(4):CD011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dessouky O, Hopkins C. Surgical versus medical interventions in CRS and nasal polyps: comparative evidence between medical and surgical efficacy. Curr Allergy Asthma Rep. 2015;15:66. [DOI] [PubMed] [Google Scholar]
- 5. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007(3):CD006394. [DOI] [PubMed] [Google Scholar]
- 6. Benninger MS, Sindwani R, Holy CE, Hopkins C. Impact of medically recalcitrant chronic rhinosinusitis on incidence of asthma. Int Forum Allergy Rhinol. 2016;6:124‐129. [DOI] [PubMed] [Google Scholar]
- 7. Soudry E, Wang J, Vaezeafshar R, Katznelson L, Hwang PH. Safety analysis of long‐term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6:568‐572. [DOI] [PubMed] [Google Scholar]
- 8. Welch KC, Thaler ER, Doghramji LL, Palmer JN, Chiu AG. The effects of serum and urinary cortisol levels of topical intranasal irrigations with budesonide added to saline in patients with recurrent polyposis after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24:26‐28. [DOI] [PubMed] [Google Scholar]


