Abstract
Given the global nature of the coronavirus disease 2019 (COVID‐19) pandemic, the need for disease detection and expanding testing capacity remains critical priorities. This review discusses the technological advances in testing capability and methodology that are currently used or in development for detecting the novel coronavirus. We describe the current clinical diagnostics and technology, including molecular and serological testing approaches, for severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) testing as well as address their advantages and limitations. Nucleic acid amplification technology for molecular diagnostics remains the gold standard for virus detection. We highlight alternative molecular detection techniques used for developing novel COVID‐19 diagnostics on the horizon. Antibody response against SARS‐CoV‐2 remains poorly understood and proper validation of serology tests is necessary to demonstrate their accuracy and clinical utility. In order to bring the pandemic under control, we must speed up the development of rapid and widespread testing through improvements in clinical diagnostics and testing technology as well as access to these tools.
Keywords: COVID‐19, SARS‐CoV‐2, RT‐PCR, serology, antibody, antigen
In December of 2019, the World Health Organization (WHO) was notified by Chinese officials of a pneumonia of unknown etiology that had affected nearly 44 individuals. On January 7, 2020, Chinese researchers isolated a novel coronavirus now known as severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) that causes the coronavirus disease 2019 (COVID‐19). Proper mitigation of continued spread of the virus relies on rapid and accurate laboratory testing to confirm positive cases. One of the first published reports of an asymptomatic transmission of disease came from a family cluster in China, 1 opening the possibility that positive patients could unknowingly be infecting others and that the number of positive cases was being underreported. Analysis of the viral load in an asymptomatic patient in China found that the levels were similar to those of symptomatic patients. 2 These findings present a challenge to successful contact tracing and exposure control. Taken together, the data underlay the importance of not only physical distancing, but more importantly expansive testing and isolation of positive cases regardless of the presence of symptoms to contain and mitigate the SARS‐CoV‐2 pandemic.
It remains critical that clinicians understand the relationships between test results from virologic and serologic testing and the performance of these diagnostic assays in order to prescribe appropriate drug therapy. Accurate interpretation and assessment of viral exposure and/or immunological response may impact pharmacotherapy decisions. As no “cure” currently exists for SARS‐CoV‐2 infection, clinical utility of validated serologic testing may have a role in clinical decision making as antibody detection may be used as an indicator of the stage of COVID‐19 progression to aid in management of the disease. Thus, it is essential that patients who are seropositive be identified in order to properly design treatments.
There are many different ways to test for SARS‐CoV‐2. In this review, we briefly describe the current and future approaches for clinical testing for COVID‐19, including molecular and serological testing as well as their benefits and limitations. We reviewed original papers published in PubMed since the COVID‐19 outbreak up to the time of writing using the following terms: COVID‐19, SARS‐CoV‐2, diagnostics, testing, real‐time reverse transcriptase polymerase chain reaction (RT‐PCR), serology, and antigen.
Nucleic Acid Amplification Test: Gold Standard RT‐PCR
Genetic information for the viral genome SARS‐CoV‐2 originally appeared in the community online resource (https://virological.org). Subsequently, updated sequences of the genome from around the world have been deposited in the viral sequence database curated by the Global Initiative on Sharing All Influenza Data (GISAID) and GenBank (accession number MN908947; National Center for Biotechnology Information [NCBI] Reference Sequence: NC_045512). Nucleic acid amplification technology (NAAT) based on polymerase chain reaction (PCR), is regarded as the “gold standard” for virus detection. The diagnosis of COVID‐19 relies on the detection of the SARS‐CoV‐2 RNA by RT‐PCR. 3 , 4 The viral RNA is detectable in nasopharyngeal and oropharyngeal swab samples as well as respiratory secretions during the acute phase of viral infection. Genetic material is extracted from patient samples and reverse transcriptase is used to make a complementary DNA strand from the viral RNA. Selective amplification of the target nucleic acid from the sample is achieved by designing target‐specific forward and reverse primers flanking regions of interest (ROIs) that are unique to the SARS‐CoV‐2 genome (Figure 1): replicase open reading frame (ORF1a/b), spike (S), envelop (E), membrane (M), and nucleocapsid (N).
Figure 1.
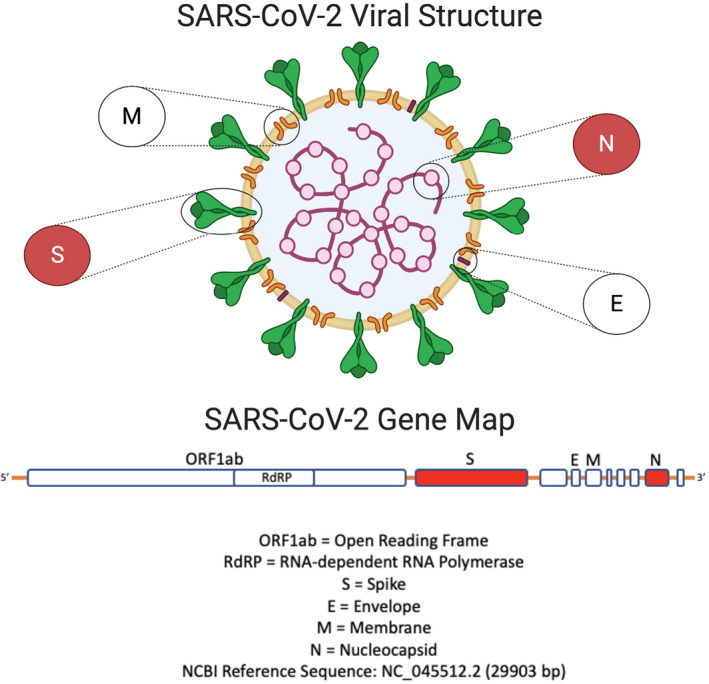
The SARS‐CoV‐2 viral structure and gene map. SARS‐CoV‐2 is a positive‐stranded RNA virus with a genome size of ∼30 kb encoding genes for non‐structural proteins (ORF1a and ORF1b) involved in replication at the 5′‐end and multiple structural proteins (S, E, M, N) downstream that make up the virus particle. Target‐specific forward and reverse primers are designed to flank regions of interest that are unique to SARS‐CoV‐2. Regions highlighted in red are believed to be those that confer viral pathogenicity. NCBI Reference Sequence: NC_045512 (GenBank accession number MN908947). Figure created with BioRender.com. E = envelop; M = membrane; N = nucleocapsid; ORF1ab = open reading frame; RdRp = RNA dependent RNA polymerase; S = spike; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2. [Color figure can be viewed at wileyonlinelibrary.com]
Understanding Target Selection and Assay Sensitivity/Specificity
The genome of SARS‐CoV‐2 has been characterized and compared with other coronaviruses to identify homology. 5 The genome of SARS‐CoV‐2 shares 89% nucleotide identity with bat SARS‐like‐CoVZXC21 and 82% with that of human SARS‐CoV. Preferred targets or ROIs include ORF1a/b, ORF1b‐nsp14, nonstructural RNA dependent RNA polymerase (RdRp), S, E, or N gene of SARS‐CoV‐2. A detailed genetic analysis of SARS‐CoV‐2 with other coronaviruses, those with low and high case fatality rates, found that pathogenicity is predicted most by the regions of the spike and nucleocapsid proteins. 6 A recent study has identified selective mutations in the SARS‐CoV‐2 genome; therefore, it is essential to avoid certain ROIs when designing primers and probes. 7 Although the mutation rate of SARS‐CoV‐2 remains to be determined, it is estimated to be moderate compared with influenza. Based on the natural viral mutation and recombination rates, targeting more than one ROI can mitigate the risk of loss of sensitivity should potential mutations arise. 8 Monitoring of genomic variations in SARS‐CoV‐2 is ongoing in case assay re‐design is needed.
Target selection can affect assay performance in terms of specificity and sensitivity as well as cross‐reactivity due to conserved regions. The validity of a test is measured by its analytical and clinical sensitivity and specificity. Analytical sensitivity is the assay’s ability to reliably detect the minimum concentration of a substance in a sample (also referred to as limit of detection) whereas analytical specificity refers to the ability to detect only the desired analyte in a specimen without cross‐reacting with other substances. Clinical sensitivity measures how accurately a test identifies positive patients who are infected. A test with 95% sensitivity will identify 95% of patients who have the disease and produces false‐negatives in 5% of patients (5 of every 100 patients who are positive test negative); a lower sensitivity test means higher false‐negative results. Clinical specificity, the inverse of sensitivity, determines how accurately a test identifies negative patients who do not have COVID‐19. A test with 95% specificity will identify 95% of patients who do not have the disease and incorrectly identify 5% of patients who test positive but actually are not infected; a lower specificity test means higher false‐positives. A test with good analytical sensitivity and specificity does not necessarily correlate with clinical sensitivity and specificity. (See section on Assay Limitations for a discussion on implications of false‐positive and negative tests.)
The WHO has established protocols for various testing kits developed around the world with a listing of the targeted viral regions (Table 1). One of the first RT‐PCR assays published for SARS‐CoV‐2 was developed by Germany Charité and targeted the RdRp, E, and N genes of the virus. The assay, designed using synthetic nucleic acid technology and in the absence of available virus isolate or original patient specimens, demonstrated that targeting the E and RdRp gene was highly sensitive, whereas the N gene was slightly less sensitive. 3 Numerous RT‐PCR assays targeting various combinations of the ROIs have since been designed to improve and maximize detection sensitivity and specificity. 9 Initially in the United States, production and validation of RT‐PCR based testing kits were completed by the Centers for Disease Control and Prevention (CDC) following federal rules and regulations. The first kits sent out to state health agencies were sidelined by a faulty reagent in the negative control and one primer set (CDC N3) that did not perform well and was later excluded from the kits. 10 Subsequent studies validated the specificity and sensitivity of the primer‐probe sets from various assays. A study conducted by the University of Washington compared the performance of their primer sets against those developed by Germany Charité 3 and the US CDC primers (CDC N1 and CDC N2) in the current CDC testing kit. The study found high specificity among all primer sets and variable sensitivity, with the CDC N2 and the Germany E‐gene sets being more sensitive than others. 11 Another study (in preprint) examined the sensitivity and efficiency of four common RT‐PCR assays developed by the US CDC, China CDC, Germany Charité, and Hong Kong University (HKU). 12 This study also found that although all primer‐probe sets can detect SARS‐CoV‐2, the most sensitive primer‐probe sets are the Germany E gene, HKU‐ORF1, HKU‐N, and US CDC N1. Whereas the US CDC N2 primers had background cross‐reactivity, it did not interfere with outcomes of the combined N1 and N2 assay when testing clinical samples from patients with COVID‐19. 12 Other studies have also compared the US CDC N1 and N2 against commercial test kits, reporting similar specificity with variable sensitivity. 13 , 14
Table 1.
World Health Organization List of Testing Protocols
| Country | Institute | Targets |
|---|---|---|
| China | China CDC | ORF1ab and N |
| Germany | Charité | RdRp, E, N |
| Hong Kong | HKU | ORF1b‐nsp14, N |
| Japan | National Institute of Infectious Diseases, Department of Virology III | Pancorona and multiple targets, S |
| Thailand | National Institute of Health | N |
| United States | CDC | Two regions in N protein |
| France | Institut Pasteur | Two regions in RdRp |
CDC = Center for Disease Control and Prevention; E = envelop protein gene; HKU = Hong Kong University; M = membrane protein gene; N = nucleocapsid protein gene; ORF = open reading frame; RdRp = RNA dependent RNA polymerase; S = spike protein gene.
To accelerate testing capacity, the US Food and Drug Administration (FDA) released updated guidance on February 29, 2020, opening up the production of diagnostic kits to state laboratories, laboratories certified under Clinical Laboratory Improvement Amendments (CLIA), and commercial diagnostic developers. 15 Clinical laboratories have the option of either using the CDC primers or other established primers/protocols listed on the WHO website, to produce their own “laboratory‐developed tests” for in‐house use. Since release of the guidance, the FDA has and continues to issue Emergency Use Authorizations (EUAs) and a comprehensive listing of all commercial diagnostic tests are updated on the FDA’s website (https://www.fda.gov/medical‐devices/coronavirus‐disease‐2019‐covid‐19‐emergency‐use‐authorizations‐medical‐devices/vitro‐diagnostics‐euas). EUAs issued to the singular developing laboratory, such as ones based at research/academic institutions and hospitals, can also be found at the same FDA website. At the time of writing, the US FDA has issued 149 individual EUAs (commercial and laboratory‐developed tests), which includes 125 molecular tests, 23 antibody tests, and 1 antigen test; a comparison of these tests is presented in Table 2. The FDA lists all individual EUAs for molecular, antibody, and antigen tests and provides links to these tests that include information on the assay platform, gene targets of the test, as well as performance characteristics (sensitivity and specificity). The WHO partners with the Foundation for Innovative New Diagnostics (FIND) for developing urgently needed diagnostic tests. FIND’s SARS‐CoV‐2 diagnostic pipeline tracks all tests commercially available or in development worldwide (https://www.finddx.org/covid‐19/pipeline/). FIND is conducting independent evaluations of SARS‐CoV‐2 testing platforms to verify the limit of detection of each test (as reported by the manufacturers) and the clinical performance of the test (https://www.finddx.org/covid‐19/sarscov2‐eval‐molecular/).
Table 2.
Comparison of In Vitro SARS‐CoV‐2 Tests Granted Emergency Use Authorizations by the US FDA
| Molecular | Antigen | Serology | |
|---|---|---|---|
| Test type | Viral | Viral | Antibody |
| Diagnostic test | Yes | Yes | No |
| Description | Nucleic acid amplification test to detect viral RNA | Detects viral proteins in the nasal cavity | Detects the presence of IgA, IgM & IgG antibodies against SARS‐CoV‐2 |
| Measure | Current infection with SARS‐CoV‐2 | Current infection with SARS‐CoV‐2 | Past exposure to SARS‐CoV‐2 |
| Platform technology | RT‐PCR, LAMP, CRISPR | Lateral flow | Lateral flow, ELISA, CIA |
| Sample type | Nasal or throat swab, saliva, bronchoalveolar lavage fluid | Nasal or throat swab | Blood draw (plasma, serum, whole blood) or finger stick |
| Testing window | Days 1–28 after symptom onset, optimal days 3–12 | Days 1–28 after symptom onset, optimal days 3–12 | IgA/IgM: from day 5 after symptom onset, optimal days 14–21; IgG: from day 14 after symptom onset up to 6 weeks |
| Result turnaround time | Same day or up to a week (depending on location); point‐of‐care option available (within 1–2 hours) | Rapid, point‐of‐care (within 15 minutes) | Same day or up to 1–3 days (depending on location); point‐of‐care option available (within 15–30 minutes) |
CIA = chemiluminescent immunoassay; CRISPR = clustered regularly interspaced short palindromic repeats; ELISA = enzyme‐linked immunosorbent assay; FDA = US Food and Drug Administration; Ig = immunoglobulin; LAMP = loop‐mediated isothermal amplification; RT‐PCR = real‐time reverse transcriptase polymerase chain reaction; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
Assay Performance and Limitations
Diagnostic testing errors can result in false‐positives and/or false‐negatives that stem from improper sample collection, testing procedural errors, and variability in assay performance (sensitivity/specificity). Diagnostic tests need to be evaluated to determine their sensitivity and specificity, ideally by comparison with a “gold standard.” The lack of a clear‐cut gold‐standard diagnostic test for SARS‐CoV‐2 (because the virus is new) makes evaluation of test accuracy challenging; therefore, assay performance needs to be compared with a reference standard. The FDA recommends selecting a comparator assay that has established high sensitivity with an internationally recognized standard or FDA SARS‐CoV‐2 Reference Panel and set the criteria at a minimum of 95% positive and negative agreement as acceptable clinical performance of the assay.
With the strategy to “test, isolate, contact trace, and quarantine,” a false‐positive result mistakenly identifies a patient as having COVID‐19 resulting in unnecessary contact tracing and quarantine. False‐negatives prevent proper contact tracing and quarantine efforts and importantly miss isolating the individual who can now infect others. Timing is critical when testing for SARS‐CoV‐2 to prevent inaccurate results. Depending on the test rendered, testing early or late in the infection cycle could lead to false‐negative results. For molecular based assays, the optimal testing window occurs near the end of the first week and into the beginning of the second week after symptom onset (Figure 2). However, positive tests are possible near the first day of symptom onset. 16 Persistent positive tests or positive tests in discharged patients after symptom resolution have been documented. In a report from South Korea CDC, 285 of a reported 447 re‐positive tests were followed; they concluded that there is no evidence that those testing positive long after symptom onset and resolution are re‐infected or still infectious. 17 The duration of viral RNA shedding is variable and depend on disease severity; prolonged viral RNA shedding of SARS‐CoV‐2 is detectable by RT‐PCR in patients recovering from COVID‐19 for up to 37 days. 18 , 19 Thus, detection of viral RNA does not necessarily reflect the presence of infectious virus and prolonged viral RNA detection following recovery does not necessarily indicate infectiousness.
Figure 2.
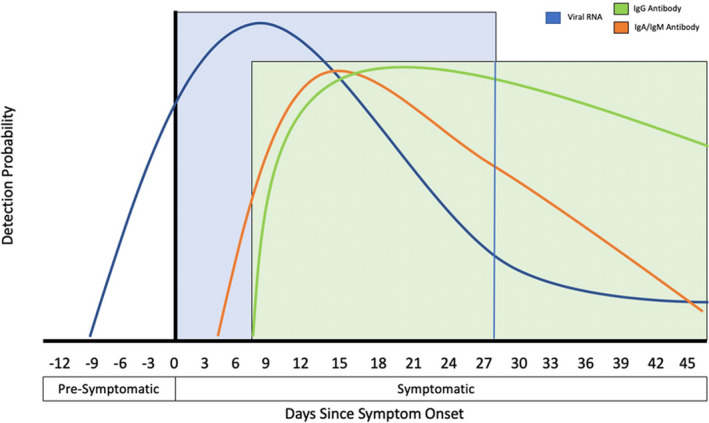
Detection probability of viral RNA or antibody (IgA, IgM, and IgG) against SARS‐CoV‐2 during the course of infection (relative to symptom onset). The testing windows of nucleic acid amplification tests (RT‐PCR, blue) and serology tests (antibody, green) are indicated. Ig = immunoglobulin; RT‐PCR = real‐time reverse transcriptase polymerase chain reaction; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2. [Color figure can be viewed at wileyonlinelibrary.com]
The implications of false‐negatives become increasingly significant as governments move forward with reopening and loosening stay at home restrictions. Recent studies have shed light and caution on the interpretation of negative test results (particularly early in the course of infection). One study demonstrated a highly variable false‐negative rate for SARS‐CoV‐2 RT‐PCR testing, suggesting 1 of 5 people suspected of having COVID‐19 may test negative based on pooled analysis of data from 7 studies. 20 Therefore, waiting 1–3 days after symptom onset can lessen the chances of a false‐negative result. If there is high clinical suspicion for infection by SARS‐CoV‐2, then serial testing is recommended to reduce the false‐negative rate. Recent studies have begun to investigate clinical performance of SARS‐CoV‐2 molecular testing to determine optimal time frames for appropriate repeat testing with 1 study suggesting 15–20 days after a positive test and the same or next 2 days after a negative test in patients with a high suspicion for infection. 21 Understanding the predictive value of NAAT with regard to time from exposure and symptom onset is important as the assay may not have been appropriately validated against a clinically meaningful reference standard for detecting SARS‐CoV‐2 in the absence of symptoms (during earlier stages of the disease) or in asymptomatic individuals. 22 The sensitivity of a test must be high for adequate containment measures and assessment of clinical sensitivity in asymptomatic patients is warranted.
Other limitations of RT‐PCR testing that can hinder use, include contamination issues, time‐consuming sample handling, 23 and variable detection of SARS‐CoV‐2 depending on the type of clinical specimen. 24 Commercial developers have been challenged to improve the real‐time PCR method and produce assays with ease of sample collection and/or processing and shorter run times to decrease turnaround times. This will enable tests to be conducted in the field at local hospitals, during doctor visits, and at mobile testing centers. In addition, the unprecedented need for testing resulted in a severe shortage of reagents and supplies, including collection swabs, transport media, extraction kits, and RT‐PCR enzyme mixtures. To circumvent shortages, suitable alternative solutions were investigated, such as comparing the use of various swabs and transport media for SARS‐CoV‐2 testing. 25 Saliva as a noninvasive specimen for detection of SARS‐CoV‐2 represents a suitable reliable alternative to help ease the global shortage of swabs for sampling and personal protective equipment used during collection. 26 , 27 The first saliva‐based test, developed and validated by Rutgers University, was issued an EAU to allow testing of saliva specimens as an alternative to samples collected through swabbing.
High Throughput, Rapid Detection, and Point‐of‐Care Diagnostics
Many of the limitations seen in available testing modalities is the necessity for expensive bulky machines, trained technical staff, and reagents. 28 These tests are most often performed in large laboratories at commercial diagnostic facilities or academic centers. Samples must therefore be sent to these laboratories, increasing both time and coordination. Improvements to the performance of the assay are available with the use of high throughput platforms, such as the cobas 6800/8800 systems, 4 which involve an automated workflow to enable high throughput testing with minimal hands‐on time, and still offering fast, reliable results (e.g., 96 results in about 3 hours or 384 results for the cobas 6800 System and 960 results for the cobas 8800 System in 8 hours). Other diagnostics that use high throughput platforms for COVID‐19 testing that have received an EUA are listed on the FDA website.
The FDA granted an EUA for the first point‐of‐care (POC) diagnostic, the Xpert Xpress SARS‐CoV‐2 test. 29 The test comes as a self‐contained kit in which a patient sample is loaded into a cartridge system and gives a readout in 45 minutes without the need of additional reagents or trained personnel. This technology allows for medical personnel in the hospital to make rapid assessments and clinical decisions. Another POC diagnostic that received the FDA’s EUA is the Accula SARS‐CoV‐2 test, a handheld device that also uses PCR technology to detect SARS‐CoV‐2 in throat and nasal swab specimens. Specimens are added to a buffer to solubilize the samples and then an aliquot is dispensed in a cassette that contains the proper controls, enzymes, and reagents, as well as a detection strip for the assay that is automated from sample extraction to nucleic acid amplification. Test results are displayed by the visualization of blue test lines on the detection strip after 30 minutes. Perhaps the most rapid POC diagnostic kit with EUA approval from the FDA is the ID NOW COVID‐19 test, which delivers positive results within 5 minutes and negative results in 13 minutes. The platform eliminates thermocycling and performs isothermal nucleic acid amplification technology with a sequence‐specific fluorescent probe readout. All of the above POC tests enable the convenience of on‐site testing and rapid detection of SARS‐CoV‐2. Limitations of these POC testing systems are that they are not built for high throughput assessments, can often be more expensive than other options, and have variable sensitivity due to the more rapid nature of the test. Studies have been conducted to examine the performance of these POC tests compared with standard RT‐PCR or in detection of low levels of viral RNA. 30 , 31 One study (comparing Xpert Xpress, ID NOW, and the ePlex systems) found that although all 3 had 100% specificity (did not exhibit false‐positive results), Xpert Xpress performed well and had the lowest limit of detection and highest sensitivity, whereas the ePlex and ID NOW had lower sensitivities. 32 Another study determined that the POC test (ID NOW) was less sensitive compared with the traditional RT‐PCR assay. 33 Furthermore, to prepare for the upcoming flu season, diagnostic assays that can simultaneously detect for SARS‐CoV‐2 and the influenza virus to determine the cause of infection will be important to clinical treatment, infection control, and community mitigation efforts. A couple of these types of tests that have already been issued FDA EUAs include the BioFire Respiratory Panel 2.1 and QIAstat‐Dx Respiratory SARS‐CoV‐2 Panel tests that can detect SARS‐CoV‐2 and 21 additional viral and bacterial respiratory pathogens, including various influenza A subtypes.
Loop‐mediated Isothermal Amplification‐based and Clustered Regularly Interspaced Short Palindromic Repeats‐based Diagnostics
There is urgent need to develop a more rapid, accurate, and POC assay to control and minimize the spread of infection in the community. Alternative molecular detection techniques have used both loop‐mediated isothermal amplification (LAMP)‐based and clustered regularly interspaced short palindromic repeats (CRISPR)‐based methods. Reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) method is another nucleic acid amplification technique that amplifies small numbers of DNA and RNA templates with high specificity and sensitivity under isothermal conditions. 34 LAMP is a one‐step amplification reaction that requires no thermocycling steps, resulting in shorter “sample‐to‐answer” times than conventional PCR. Thus, the assay is ideally suited for field‐based nucleic acid diagnostics due to rapid results (usually 30–60 minutes) and minimal requirements for expensive instrumentation or reagents. Previous RT‐LAMP assays with improved specificity, visualization techniques, or readouts have been developed for detecting Middle East respiratory syndrome coronavirus (MERS)‐CoV and SARS‐CoV. Several LAMP‐based diagnostics are in the pipeline for detecting SARS‐CoV‐2 that have been developed by laboratories worldwide, including South Korea, 35 , 36 China, 37 , 38 , 39 and the United Kingdom, 40 as well as in the United States with LAMP and CRISPR technology developed in combination (discussed in the next section). A rapid colorimetric assay using RT‐LAMP is being developed for the qualitative detection of virus directly from saliva. 41
The powerful genome editing technology known as CRISPR has also been recently exploited to develop rapid, inexpensive, POC diagnostics for COVID‐19 testing. The RNA‐targeting CRISPR associated enzyme Cas13 can be programmed to target and destroy single stranded RNA viruses. 42 A platform termed specific high‐sensitivity enzymatic reporter unlocking (SHERLOCK) was developed to combine isothermal preamplification with Cas13 to detect single molecules of RNA or DNA. 43 , 44 The CRISPR‐Cas13‐based SHERLOCK COVID‐19 detection protocol searches for unique nucleic acid targets (SARS‐CoV‐2 ORF1ab and S genes) and uses a dipstick as the visual readout in less than an hour. 45 This CRISPR‐based methodology tags the target SARS‐CoV sequences with a fluorescent probe. When the Cas13 enzyme recognizes the specific genetic sequences, it becomes activated to cut up all nearby RNA, including a “reporter” molecule added to the reaction, resulting in a signal, such as a measurable increase in fluorescence, which reveals the presence of viral genetic material. An adaptation that combines this detection methodology with microfluidic chips was recently developed and called the Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic acids (CARMEN) platform. A single CARMEN‐Cas13 chip can be scaled to either detect a single type of virus in more than 1000 samples at a time or 160 different viruses in a small number of samples. 46 Another similar CRISPR‐based test uses the DNA endonuclease‐targeted CRISPR trans reporter (DETECTR) platform combining RT‐LAMP with Cas12. 47 The SARS‐CoV‐2 DETECTR protocol uses the N and E genes as targets with a similar visual lateral flow strip readout that enables viral RNA detection within 45 minutes. 48 At the time of this writing, the SHERLOCK CRISPR SARS‐CoV‐2 is the first LAMP/CRISPR‐based diagnostic to receive FDA EUA status.
Antigen Detection Testing
The antigen test for SARS‐CoV‐2 is a diagnostic test designed for rapid detection of viral proteins (antigens). Although the main advantage of these antigen tests is the speed of the test, they are often plagued with inaccurate results and have lower sensitivity and specificity than nucleic acid assays. Previous rapid antigen tests for influenza (H1N1) and respiratory syncytial virus were found to be less sensitive compared with RT‐PCR. 49 , 50 Development of antibodies that are specific to viral antigens is often difficult and time‐consuming and antigen tests must undergo rigorous quality control measures to ensure specificity and sensitivity. Although antigen tests are very specific for the virus, they cannot detect all active infections and thus have a higher chance of false‐negatives than RT‐PCR. The FDA issued an EUA to the first antigen assay, the Sofia 2 SARS Antigen FIA test, which uses immunofluorescence‐based lateral flow technology in a sandwich design that is used to detect the viral nucleocapsid protein in nasopharyngeal and nasal swab specimens with test results in 15 minutes. Future research on the clinical performance of antigen testing compared to NAAT is highly encouraged to determine their diagnostic utility.
Serology Testing
Outside of nucleic acid amplification‐based diagnostics, serological or antibody tests for COVID‐19 is another promising testing modality. Both molecular and serological tests are intended for patients at different stages of the disease process with RT‐PCR for detection of current active infection and serology tests for later stages because these assays detect the presence of SARS‐CoV‐2 antibodies produced by the body’s humoral immune system. Serologic testing enables the understanding of how patients produce antibodies to SARS‐CoV‐2, and assays can detect immunoglobulin (Ig)A, IgM, IgG, or total antibody. Profiling of early humoral response in one study of patients with COVID‐19 determined that IgA and IgM antibodies can be detected as early as 5 days after new infection, with higher levels detected in the second and third weeks. 51 , 52 Other studies detected optimal IgM antibodies toward the end of the first week that peaked during the third week after symptom onset, and may correspond to the emergence of negative results by RT‐PCR tests as the viral load is cleared (Figure 2). 16 , 19 , 51 , 53 Another report determined earlier IgA seroconversion than IgM 54 or that IgA is detected at higher levels than IgM. 55 IgG antibodies become detectable later in the infection course around 7–14 days after symptom onset and levels remained relatively high until 6 weeks. 51 , 52 Although the data remain premature, a small study of 285 patients with COVID‐19 reported that 100% of patients tested positive for IgG within 20 days after symptom onset. Seroconversion for IgG and IgM occurred simultaneously or sequentially between the third and fourth weeks, and both levels plateaued within 6 days after seroconversion. 56 Asymptomatic patients may seroconvert later in the course of infection or may not at all. 57 Another study found that asymptomatic patients have significantly longer duration of viral shedding, lower IgG, and neutralizing serum antibody levels compared with the symptomatic group, suggesting that asymptomatic patients may mount a weaker antibody response and immunity may diminish within months of infection. 58 Although these studies demonstrate variable antibody response, IgA/IgM antibodies seem to develop during the earlier stages (indicative of recent exposure) 59 whereas IgG antibodies show up later in the course of SARS‐CoV‐2 infection. The association between antibody response and clinical course remains to be determined, and recent studies have proposed that antibody detection may be used as an indicator of the stage of COVID‐19 progression to aid in management of the disease. 60 The dynamics of humoral response in different populations (mild vs severe illness) and understanding duration of immunity to SARS‐CoV‐2, as well as the assessment of neutralizing antibodies (and its relationship to protection from future infection) are ongoing investigations.
There are two types of antibody tests: binding antibody detection and neutralizing antibody detection. The former uses purified proteins of SARS‐CoV‐2 to determine antibody binding coupled with one of the following platforms: colloidal gold‐based immunochromatographic assay (also known as lateral flow immunoassay, which is a POC test), chemiluminescent immunoassay (CIA), and enzyme‐linked immunosorbent assay (ELISA). Neutralizing antibodies prevent virus infection in vitro, and there are currently no FDA authorized neutralization tests for SARS‐CoV‐2. The FDA issued an EUA to the first antibody test, the Cellex qSARS‐CoV‐2 IgG/IgM Rapid Test. The lateral flow assay detects IgM and IgG antibodies to SARS‐CoV‐2 in serum, plasma, or whole blood specimens (not finger stick specimens), and results are read in 15–20 minutes. The test is conducted by CLIA laboratories and requires reporting of positive results to the public health authorities. Subsequent antibody tests have been granted EUAs using various platforms, including ELISAs and CIAs, and all authorized tests are qualitative rather than quantitative. A comprehensive listing of all commercially marketed antibody tests granted EUAs are updated on the FDA and FIND websites on a rolling basis.
Understanding Target Selection, Assay Sensitivity/Specificity, and Limitations
Serology tests are currently being developed for the detection of antibodies (in blood plasma or serum) to two main antigenic targets, the N protein and S protein, including subunits S1 and S2 as well as the receptor‐binding domain (RBD) of SARS‐CoV‐2. The target selection determines cross‐reactivity and specificity with N being more conserved across coronaviruses than S and within S, the RBD is more conserved than either subunit.
Proper serology testing requires understanding the performance characteristics and limitations of these tests. Serological tests have varying levels of specificity and sensitivity and can detect past infections in those who are RT‐PCR negative. False‐positives can result from cross‐reactivity with preexisting antibodies from previous infections, such as other coronaviruses (229E, NL63, OC43, and HKU1 that cause the common cold; SARS‐CoV or MERS‐CoV; an antibody test with low specificity); whereas negative results may be due to antibodies that have not yet formed during the early stages of infections. Initial studies have shown that N and S1 protein ELISAs could detect SARS‐CoV‐2 with high specificity; RBD and N protein ELISAs are more sensitive than S1 assays in detecting antibodies in patients with mild infections. 61 Researchers also found that antibodies to N protein are more sensitive than to S protein during early infection using a liquid phase immunoassay called luciferase immunoprecipitation systems. 62 A meta‐analysis was recently conducted to investigate the performance of all available antibody tests and found that tests using the S antigen are more sensitive than N antigen‐based tests. A combined IgG/IgM test displayed better sensitivity than measuring either antibody type alone with ELISA tests being more sensitive than lateral flow immunoassays. 63 A recent study found high sensitivity and specificity of the RBD for antibody detection and its strong association with SARS‐CoV‐2 neutralizing antibodies. 59 Studies are ongoing to determine the clinical performance characteristics of various antibody tests across different platforms. 64 , 65
The prevalence of SARS‐CoV‐2 antibody positive individuals in the US population remains to be determined and is based on the different rates of infection across various locations and populations. The positive and negative predictive values (PPV and NPV) of antibody tests will affect the overall outcome of testing and are determined by the prevalence of the disease and the sensitivity/specificity of the test. PPV is the probability that a patient who tests positive truly has the disease and NPV is the chance that one who tests negative has not been infected. In the setting of low prevalence of SARS‐CoV‐2 infection, even a highly specific test can give a large number of false‐positives; thus, the PPV will be low. The FDA has summarized the expected performance of the tests it has authorized based on the information the FDA reviewed when deciding whether or not to grant these tests an EUA and assuming a prevalence of 5% for PPV and NPV calculations. We refer the reader to the FDA’s website for the performance characteristics of the individual tests that have received EUA status (https://www.fda.gov/medical‐devices/emergency‐situations‐medical‐devices/eua‐authorized‐serology‐test‐performance).
Antibody response against SARS‐CoV‐2 remains poorly understood and the clinical utility of tests is unclear until the assays are properly validated to demonstrate their accuracy. Massive efforts are currently underway to conduct large‐scale validation studies on the performance of these assays, which is critical before they can be used in seroprevalence studies for disease surveillance. A collaborative effort by the FDA, National Institutes of Health (NIH), CDC, and Biomedical Advanced Research and Development Authority (BARDA) is currently underway to conduct the performance assessments and establish the validity of serological tests against a well‐characterized set of clinical samples collected before and during the pandemic and correlate them with neutralization assays. The validation dataset is published on the openFDA website (https://open.fda.gov/apis/device/covid19serology/). Properly validated accurate tests can then potentially be used in the future as a public health surveillance tool to monitor the epidemiological spread of COVID‐19, as a risk assessment tool to screen for immunity, or as a screening tool to identify donor patients who have antibodies for convalescent plasma treatment.
At‐Home Sample Collection
Some companies have been developing at‐home sample packs. These test kits would include the necessary components and instructions to properly obtain a nasal swab sample while at home. The kits would then be mailed to a central laboratory for testing. On April 21, 2020, the FDA authorized the first molecular diagnostic test with a home collection option for LabCorp’s RT‐PCR assay to permit testing of samples self‐collected by patients at home using the company’s home collection kit. Subsequently, Rutgers University also received EUA designation for its at‐home saliva self‐collection assay. Everlywell’s COVID‐19 Test Home Collection Kit is the first standalone sample collection kit (for self‐collecting a nasal sample at home) to be granted an EUA and can be paired with any authorized NAAT at any laboratory.
At‐home collection provides an additional solution that limits exposure and keeps patients isolated. However, there are still some concerns that may need to be addressed. The availability of nasopharyngeal swab supplies may pose an issue if the test needs to continue to expand and swabs used for in‐person testing are in short supply in some regions. Companies would need to develop alternative methods of sample collection, such as the use of oral swabs or finger stick whole blood serology tests to provide some relief for the swab‐based tests. Improper self‐collection may affect specimen quality and subsequent test results. At‐home sample collection may encounter issues with poor sampling and ongoing studies will need to examine whether samples (saliva, dried blood spot, and swabs) self‐collected by patients at home and monitored by a provider through telehealth are sufficient specimens for molecular and serology SARS‐CoV‐2 testing. 66 Additional limitations to these tests are the time incurred in shipping the test kits as well as the time for the laboratory assessment.
Conclusions
In the face of a pandemic, testing, contact tracing, and isolation are all important parameters to contain the spread of COVID‐19. Although much is unknown about the new coronavirus, it is with certainty that SARS‐CoV‐2 infection with be a recurring disease. Mounting evidence from recent studies demonstrate the existence of viral transmission from presymptomatic and asymptomatic patients. 67 In the latter scenario, expansive testing remains a priority on the global level. Widespread testing must increase in order to effectively enact isolation procedures and contact tracing measures to detect asymptomatic infections in order to break transmission chains and attempt to flatten the curve. The NIH (in partnership with the FDA, CDC, and the Biomedical Advanced Research and Development Authority) launched a national innovation initiative for COVID‐19 diagnostics called the Rapid Acceleration of Diagnostics (RADx). The RADx initiative is aimed at speeding innovation, development, and commercialization of COVID‐19 testing technologies with the overall goal of speeding up the development of rapid and widespread testing. The National Cancer Institute (in collaboration with the National Institute of Allergy and Infectious Diseases and other NIH and the US Department of Health and Human Services [DHHS] partnerships) recently established the Serological Sciences Network for COVID‐19 (SeroNet) to address the urgent need to increase our understanding of the mechanisms driving the serological, humoral, and cellular immune responses to SARS‐CoV‐2 infection in order to inform the development of novel serological tests. Moreover, the US DHHS has authorized pharmacists, under the Public Readiness and Emergency Preparedness Act, to order and administer SARS‐CoV‐2 testing. In the foreseeable future, pharmacies and pharmacists, being the most accessible health care providers, can greatly contribute to expanding testing efforts. In order for the United States and the global community to continue having a positive effect on the infection curve and bring the pandemic under control, we must increase our testing capacity, which can only be made possible through improvements in clinical diagnostics and testing technology as well as access to these tools.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US Government.
Conflict of interest: The authors have declared no conflicts of interest for this article.
Funding information: This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (ZIA BC 011974).
References
- 1. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis 2020;20:410–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill 2019;2020:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pfefferle S, Reucher S, Nörz D, Lütgehetmann M. Evaluation of a quantitative RT‐PCR assay for the detection of the emerging coronavirus SARS‐CoV‐2 using a high throughput system. Euro Surveill 2020;25(9):2000152. 10.2807/1560-7917.es.2020.25.9.2000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gussow AB, Auslander N, Faure G, Wolf YI, Zhang F, Koonin EV. Genomic determinants of pathogenicity in SARS‐CoV‐2 and other human coronaviruses. Proc Natl Acad Sci USA 2020;117(26):15193–15199. 10.1073/pnas.2008176117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C, Liu Z, Chen Z, et al. The establishment of reference sequence for SARS‐CoV‐2 and variation analysis. J Med Virol 2020;92:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penarrubia AL, Ruiz M, Porco R, et al. Multiple assays in a real‐time RT‐PCR SARS‐CoV‐2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID‐19 outbreak. Int J Infect Dis 2020;97:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan JF‐W, Yip CC‐Y, To KK‐W, et al. Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific COVID‐19‐RdRp/Hel real‐time reverse transcription‐pcr assay validated in vitro and with clinical specimens. J Clin Microbiol 2020;58(5). 10.1128/jcm.00310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen J. The United States badly bungled coronavirus testing—but things may soon improve. Science 2020. 28 February 2020. https://www.sciencemag.org/news/2020/02/united‐states‐badly‐bungled‐coronavirus‐testing‐things‐may‐soon‐improve. [Google Scholar]
- 11. Nalla AK, Casto AM, Huang M‐LW, et al. Comparative performance of SARS‐CoV‐2 detection assays using seven different primer‐probe sets and one assay kit. J Clin Microbiol 2020;58(6). 10.1128/jcm.00557-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vogels CBF, Brito AF, Wyllie AL, et al. . Analytical sensitivity and efficiency comparisons of SARS‐CoV‐2 RT–qPCR primer–probe sets. Nat Microb 2020. 10.1038/s41564-020-0761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhen W, Manji R, Smith E, Berry GJ. Comparison of four molecular in vitro diagnostic assays for the detection of SARS‐CoV‐2 in nasopharyngeal specimens. J Clin Microbiol 2020;58(8). 10.1128/jcm.00743-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lieberman JA, Pepper G, Naccache SN, Huang M‐Li, Jerome KR, Greninger AL. Comparison of commercially available and laboratory‐developed assays for in vitro detection of SARS‐CoV‐2 in clinical laboratories. J Clin Microbiol 2020;58(8). 10.1128/jcm.00821-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Policy for Diagnostic Tests for Coronavirus Disease‐2019 during the Public Health Emergency. Immediately in Effect Guidance for Clinical Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff: US Food and Drug Administration; 2020.
- 16. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA 2020;323(22):2249. [DOI] [PubMed] [Google Scholar]
- 17. Findings from investigation and analysis of re‐positive cases. Korean Centers for Disease Control and Prevention.
- 18. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis 2020;20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature 2020;581:465–9. [DOI] [PubMed] [Google Scholar]
- 20. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction–based SARS‐CoV‐2 tests by time since exposure. Ann Intern Med 2020. 10.7326/m20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green DA, Zucker J, Westblade LF,et al . Clinical performance of SARS‐CoV‐2 molecular tests. J Clin Microbiol 2020;58(8). 10.1128/jcm.00995-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS‐CoV‐2 infection — challenges and implications. N Engl J Med 2020. 10.1056/nejmp2015897 [DOI] [PubMed] [Google Scholar]
- 23. Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID‐19). Clin Chem Lab Med 2020;58(7):1070–6. [DOI] [PubMed] [Google Scholar]
- 24. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermeiren C, Marchand‐Senécal X, Sheldrake E, et al. Comparison of Copan ESwab and FLOQSwab for COVID‐19 diagnosis: Working around a supply shortage. J Clin Microb 2020;58(6). 10.1128/jcm.00669-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. To KK‐W, Tsang OT‐Y, Yip CC‐Y, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020. 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect 2020;81(1):e45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen T, Duong Bang D, Wolff A. Novel coronavirus disease (COVID‐19): paving the road for rapid detection and point‐of‐care diagnostics. Micromachines 2019;2020:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loeffelholz MJ, Alland D, Butler‐Wu SM, et al. Multicenter evaluation of the cepheid Xpert Xpress SARS‐CoV‐2 test. J Clin Microb 2020;58(8). 10.1128/jcm.00926-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lowe CF, Matic N, Ritchie G, et al. Detection of low levels of SARS‐CoV‐2 RNA from nasopharyngeal swabs using three commercial molecular assays. J Clin Virol 2020;128:104387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran A, Beavis KG, Matushek SM, et al. Detection of SARS‐CoV‐2 by use of the cepheid Xpert Xpress SARS‐CoV‐2 and roche cobas SARS‐CoV‐2 assays. J Clin Microb 2020;58(8). 10.1128/jcm.00772-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample‐to‐answer platforms for detection of SARS‐CoV‐2. J Clin Microb 2020;58(8). 10.1128/jcm.00783-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrington A, Cox B, Snowdon J, et al. Comparison of Abbott ID now and Abbott m2000 methods for the detection of SARS‐CoV‐2 from nasopharyngeal and nasal swabs from Symptomatic patients. J Clin Microb 2020;58(8). 10.1128/jcm.00798-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Notomi T, Mori Y, Tomita N, Kanda H. Loop‐mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 2015;53:1–5. [DOI] [PubMed] [Google Scholar]
- 35. Baek YH, Um J, Antigua KJC, et al. Development of a reverse transcription‐loop‐mediated isothermal amplification as a rapid early‐detection method for novel SARS‐CoV‐2. Emerg Microbes Infect 2020;9(1):998–1007. 10.1080/22221751.2020.1756698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park G‐S, Ku K, Baek S‐H, et al. Development of reverse transcription loop‐mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). J Mol Diagn 2020;22(6):729–735. 10.1016/j.jmoldx.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu R, Wu X, Wan Z, Li Y, Jin X, Zhang C. A novel reverse transcription loop‐mediated isothermal amplification method for rapid detection of SARS‐CoV‐2. Int J Mol Sci 2020;21(8):2826. 10.3390/ijms21082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu L, Wu S, Hao X, et al. Rapid detection of COVID‐19 coronavirus using a reverse transcriptional loop‐mediated isothermal amplification (RT‐LAMP) diagnostic platform. Clin Chem 2020;66(7):975–977. 10.1093/clinchem/hvaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan C, Cui J, Huang L, et al. Rapid and visual detection of 2019 novel coronavirus (SARS‐CoV‐2) by a reverse transcription loop‐mediated isothermal amplification assay. Clin Microbiol Infect 2020;26(6):773–779. 10.1016/j.cmi.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang WE, Lim B, Hsu CC, et al. RT‐LAMP for rapid diagnosis of coronavirus SARS‐CoV‐2. Microb Biotechnol 2020;13(4):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lalli MA, Chen X, Langmade SJ, et al. Rapid and extraction‐free detection of SARS‐CoV‐2 from saliva with colorimetric LAMP. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 42. Gootenberg JS, Abudayyeh OO, Lee JW, et al. Nucleic acid detection with CRISPR‐Cas13a/C2c2. Science 2017;356:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018;360:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 2019;14:2986–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng Zhang OOA, Gootenberg JS. A protocol for detection of COVID‐19 using CRISPR diagnostics; 2020. https://www.broadinstitute.org/files/publications/special/COVID‐19%20detection%20(updated).pdf
- 46. Ackerman CM, Myhrvold C, Thakku SG, et al. Massively multiplexed nucleic acid detection with Cas13. Nature. 2020;582(7811):277–282. 10.1038/s41586-020-2279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen JS, Ma E, Harrington LB, et al. CRISPR‐Cas12a target binding unleashes indiscriminate single‐stranded DNase activity. Science 2018;360:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Broughton JP, Deng X, Yu G, et al. CRISPR‐Cas12‐based detection of SARS‐CoV‐2. Nat Biotechnol 2020;38(7):870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al Johani SM, Al Balawi M, Al Alwan B, Al Hefdhi R, Hajeer A. Validity of two rapid point of care influenza tests and direct fluorescence assay in comparison of real time PCR for swine of origin Influenza virus. J Infect Public Health 2011;4:7–11. [DOI] [PubMed] [Google Scholar]
- 50. Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta‐analysis. J Clin Microbiol 2015;53:3738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis 2020. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID‐19. Cell Mol Immunol 2020;17(7):773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis 2020. 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu H‐q, Sun B‐q, Fang Z‐f, et al. Distinct features of SARS‐CoV‐2‐specific IgA response in COVID‐19 patients. Eur Respir J 2020:2001526. https://oi.org/10.1183/13993003.01526‐2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Padoan A, Sciacovelli L, Basso D, et al. IgA‐Ab response to spike glycoprotein of SARS‐CoV‐2 in patients with COVID‐19: a longitudinal study. Clin Chim Acta 2020;507:164–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Long Q‐X, Liu B‐Z, Deng H‐J, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med 2020;26(6):845–848. 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 57. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Emerg Microbes Infect 2020;9:833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Long Q‐X, Tang X‐J, Shi Q‐L, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med 2020. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 59. Premkumar L, Segovia‐Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Sci Immunol 2020;5(48):eabc8413. 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients. Emerg Infect Dis 2020;26(7):1478–1488. 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients With coronavirus disease 2019. J Infect Dis 2020;222(2):206–213. 10.1093/infdis/jiaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kontou PI, Braliou GG, Dimou NL, Nikolopoulos G, Bagos PG. Antibody tests in detecting SARS‐CoV‐2 infection: A meta‐analysis. Diagnostics 2020;10(5):319. 10.3390/diagnostics10050319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Theel ES, Harring J, Hilgart H, Granger D. Performance characteristics of four high‐throughput immunoassays for detection of IgG antibodies against SARS‐CoV‐2. J Clin Microbl 2020;58(8). 10.1128/jcm.01243-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Clinical performance of different SARS‐CoV‐2 IgG antibody tests. J Med Virol 2020. 10.1002/jmv.26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sullivan PS, Sailey C, Guest JL, et al. Detection of SARS‐CoV‐2 RNA and antibodies in diverse samples: Protocol to validate the sufficiency of provider‐observed, home‐collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill 2020;6(2):e19054. 10.2196/19054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furukawa NW, Brooks JT, Sobel J. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic. Emerg Infect Dis 2020;26(7). 10.3201/eid2607.201595 [DOI] [PMC free article] [PubMed] [Google Scholar]


