Abstract
Obesity and COVID‐19 are both worldwide epidemics now. There may be some potential relationships between them, but little is known. This study was done to explore this relationship through literature search, systematic review, and meta‐analysis. Pubmed, Embase, WOS, Cochrane, CNKI, Wanfang, and Sinomed databases were searched to collect literature concerning obesity and COVID‐19. Systematic review and meta‐analysis were conducted after literature screening, quality assessment, and data extraction. A total of 180 articles were initially searched after duplicate removal, and 9 were finally included in our analysis. Results show that severe COVID‐19 patients have a higher body mass index than non‐severe ones (WMD = 2.67; 95% CI, 1.52‐3.82); COVID‐19 patients with obesity were more severely affected and have a worse outcome than those without (OR = 2.31; 95% CI, 1.3‐4.12). Obesity may aggravate COVID‐19.
Keywords: COVID‐19, meta‐analysis, obesity, risk factor, systematic review
Highlights
Severe COVID‐19 patients have higher BMI than non‐severe ones.
COVID‐19 patients with obesity were more severe than those without.
Obesity may aggravate COVID‐19 disease.
1. INTRODUCTION
The 2019 novel coronavirus disease (COVID‐19) is a kind of coronavirus infection and was first reported on 31 December 2019. It has spread all over the world, infected more than 2.8 million people and claimed 200 000 deaths as of 26 April 2020. The mortality rate of COVID‐19 patients increases when complicated with diabetes, cardiovascular disease, hypertension, and other underlying diseases. 1 , 2 Considering large numbers of elderly people with underlying diseases in the outbreak areas, the situation for epidemic prevention and control is serious. 3 , 4
Obesity is very prevalent in the United States and Europe, with an incidence higher than 40%. 5 , 6 It could lead to diabetes, cardiovascular disease, and tumors, which are all associated with susceptibility or higher mortality of COVID‐19. 7 , 8 , 9
Studies have indicated that obese COVID‐19 patients are more likely to receive mechanical ventilation and have a higher mortality rate. 10 These facts remind us that obesity may be closely related to the aggravation of COVID‐19. At the same time, two earlier reports have suggested no difference in the body mass index(BMI) between severe and non‐severe groups. 11 , 12 This contradicts the speculation above. To elucidate the relationship between obesity and COVID‐19, this study was done to conduct a systematic review and meta‐analysis on this topic by searching the existing literature.
2. MATERIALS AND METHODS
2.1. Search strategy
Pubmed, Embase, Cochrane Library, Web of Science were searched for English articles published before 22 April 2020 and CNKI, Wanfang, Sinomed for Chinese articles. Medical Subject Heading (MeSH) and keywords were used together, including COVID‐19, coronavirus disease 2019, 2019‐nCoV infection, 2019‐nCoV disease, 2019 novel coronavirus disease, 2019 novel coronavirus infection, SARS‐CoV‐2 infection and obesity, Body Mass Index, Quetelet Index, Quetelet's Index, BMI, weight circumference. This approach was also combined with a manual search of references in all selected studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) COVID‐19 patients were confirmed by nasopharyngeal swab or sputum PCR; (ii) comparison was made between obese and nonobese COVID‐19 patients, or between severe and non‐severe COVID‐19 patients; (iii) outcome indicators were severity of COVID‐19 or obesity; (iv) articles were published in English or Chinese. Also, the exclusion criteria were as follows: (i) review articles, basic research, case reports, guidelines, a consensus of opinions or other unrelated topics; (ii) those not referring to the association between obesity and COVID‐19; (iii) those without quantitative data referring to the association. Literature screening was performed by two investigators independently (Jun Yang and Jiahui Hu). Disagreements were resolved by consensus.
2.3. Data extraction and quality assessment
Baseline characteristics and target parameters were extracted from the selected articles. For each study, the author, year of publication, country, underlying disease, clinical type, gender composition, and the number of patients of each type were extracted. BMI and severity of COVID‐19 were chosen as our target parameters. The Newcastle‐Ottawa Scale (NOS) was used to evaluate the quality of each observational study. The NSO scoring criteria requires the experimenter to select the subjects reasonably (4 points), to make the subjects comparable between groups (2 points) and to evaluate the outcome index scientifically (3 points). A study with 6 points or higher will have good quality. Two investigators (Jun Yang and Jiahui Hu) carried out the work individually.
2.4. Statistical analysis
BMI values of non‐severe and severe groups were extracted from each relevant article. The weighted mean deviation (WMD) and 95% confidence interval (95% CI) of each relevant study were calculated by using Stata 16.0 software. The number of patients with or without obesity in severe or non‐severe COVID‐19 groups was extracted. The OR and 95% CI of each relevant study were calculated with SPSS 22.0 software. Data integration and forest map drawing were performed with Stata 16.0. q test and I 2 test were used to judge the heterogeneity. If the P value was less than .1 or I 2 greater than 0.5, the heterogeneity would be considered large. Then, the random effect model would be used to combine the numerical values of each study. Sensitivity analysis was used to identify the source of heterogeneity. Otherwise, the fixed‐effect model was used. Funnel plots were drawn to evaluate the publication bias of the included literature. All P values were bilateral, and P < .05 was considered significant (Figure 1).
Figure 1.
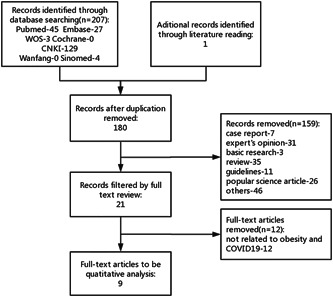
Flow chart of included studies
3. RESULTS
3.1. Study characteristics
We found 180 articles without duplication for eligibility and included 9 for systematic review and meta‐analysis after screening. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Among those included, six 10 , 11 , 12 , 15 , 16 , 18 were retrospective case‐control studies and four 10 , 13 , 14 , 17 were retrospective cohort studies. It was worth noting that one of the papers contain two research approaches and involved both types of integrated analysis. 10 The quality of study design among the selected studies was median, with a median quality score of 6. Seven of the nine studies were conducted in China, 11 , 12 , 14 , 15 , 16 , 17 , 18 while one was done in France 10 and one in the United States. 13 They were published between February and April 2020. A total of 4444 participants were enrolled in our study. Five of the nine studies had fewer than 100 subjects. 11 , 12 , 13 , 17 , 18 Two studies, one from the United States 13 and the other from France, 10 used 30 kg/m2 as diagnostic thresholds for obesity, while the remaining two studies from China took 24 17 and 25 kg/m2, 14 respectively. Most of the studies chose COVID‐19 patients as subjects, while two identified those patients with complications like cardiovascular disease 15 and metabolic associated fatty liver disease, 14 respectively. Age differed among the studies, ranging from younger than 40 years old to older than 60 years old. The detailed characteristics are presented in Table 1.
Table 1.
Main characteristics of included articles
| Non‐severe | Severe | NOS score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Ethnicity | Obesity | Disease | No, M/F | Age, y | No, M/F | Age, y | |
| Wang | 2020 | China | Asian | … | COVID‐19 | 54 (22/32) | ≥60.4 | 18 (10/8) | ≥60.7 | 6 |
| Wu | 2020 | China | Asian | … | COVID‐19 | 197 (106/91) | 37.55 ± 17.10 | 83 (45/38) | 63.04 ± 10.20 | 5 |
| Liu | 2020 | China | Asian | … | COVID‐19 | 26 | … | 4 | … | 5 |
| Xiang | 2020 | China | Asian | … | COVID‐19 | 40 (25/15) | 40.6 ± 14.3 | 9 (8/1) | 53.0 ± 14.0 | 6 |
| Peng | 2020 | China | Asian | … | CVD + COVID‐19 | 96 (44/52) | 58.2 ± 7.3 | 16 (9/7) | 61.5 ± 9.4 | 5 |
| Lighter | 2020 | USA | Caucasian | ≥30 kg/m2 | COVID‐19 | 2245 | … | 1370 | … | 5 |
| Simonnet | 2020 | France | Caucasian | >30 kg/m2 | COVID‐19 | 65 | … | 59 | … | 6 |
| Zheng | 2020 | China | Asian | >25 kg/m2 | MAFLD + COVID‐19 | 21 (17/4) | 18‐44:15;45‐64:6 | 45 (32/13) | 18‐44:24; 45‐64:16; ≥65:5 | 7 |
| Wang | 2020 | China | Asian | >24 kg/m2 | COVID‐19 | 52 | … | 44 | … | 6 |
Abbreviations: COVID‐19, coronavirus disease; CVD, cardiovascular disease; F, female; M, male; MAFLD, metabolic associated fatty liver disease; No, number; NOS score, Newcastle‐Ottawa Scale score.
3.2. BMI in non‐severe vs severe
Six studies have compared BMI in severe and non‐severe COVID‐19 patients. We integrated these results and found a moderate heterogeneity among them (I 2 = 63.8%, P = .017). Then, we chose the random‐effects model (REM) to combine the effect quantity. The results show that severe patients have a higher BMI, with a pooled WMD of 2.67 (95% CI, 1.52‐3.82), than non‐severe ones (Figure 2A).
Figure 2.
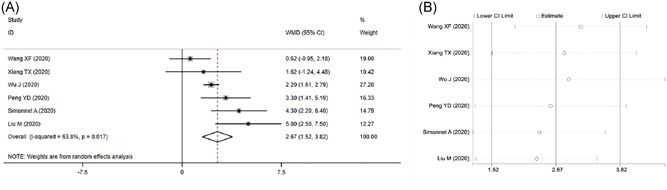
Meta‐analysis of BMI in severe vs non‐severe COVID‐19. A, Forest plot. B, Sensitivity analysis
Considering the heterogeneity among six included studies, we carried out a sensitivity analysis to verify its stability. The results show that no study changed the overall heterogeneity, which was stable (Figure 2B).
As sample size and disease severity vary among these studies, we carried out a subgroup analysis to identify possible disturbances. The results indicate that sample size and disease severity seem to play less of a role in the overall heterogeneity (Table 2).
Table 2.
Summary of the subgroup analysis results
| WMD (95% CI) | Heterogeneity | |||||
|---|---|---|---|---|---|---|
| Analysis | N | Reference | Fixed‐effect model | Randon‐effect model | I 2, % | P |
| Included cases | 6 | 10‐12, 15, 16, 18 | 2.33 (1.83, 2.82) | 2.67 (1.52, 3.82) | 63.8 | .017 |
| <100 | 3 | 11, 12, 18 | 1.81 (0.60, 3.01) | 2.32 (−0.39, 5.03) | 76.5 | .014 |
| >100 | 3 | 10, 15, 16 | 2.43 (1.89, 2.98) | 2.95 (1.69, 4.21) | 54.9 | .109 |
| Severity of disease | 6 | 10‐12, 15, 16, 18 | 2.33 (1.83, 2.82) | 2.67 (1.52, 3.82) | 63.8 | .017 |
| Non‐severe vs severe | 4 | 11, 12, 16, 18 | 2.12 (1.59, 2.65) | 2.18 (0.76, 3.61) | 66.1 | .031 |
| Noncritical vs critical | 2 | 10, 15 | 3.75 (1.83, 2.82) | 3.76 (2.34, 5.15) | 0.0 | .488 |
Abbreviations: N, number of studies; WMD, weighted mean difference.
As the number of included papers in this part was less than 10 articles, we didn't carry out meta‐regression analysis.
3.3. Disease severity in nonobese vs obese
Four studies classified the subjects according to whether they were obese or not. We extracted data from those studies, calculated the OR value, and integrated the effect quantity. The meta‐analysis of disease severity in nonobese vs obese groups included these four studies. Preliminary analysis shows that a moderate heterogeneity stands out among these studies(I 2 = 61.6%, P = .05). We used REM to integrate effect quantity. The results show that the obese patients had a more severe outcome, with a pooled OR of 2.31 (95% CI, 1.3‐4.12), when compared to nonobese ones (Figure 3A).
Figure 3.
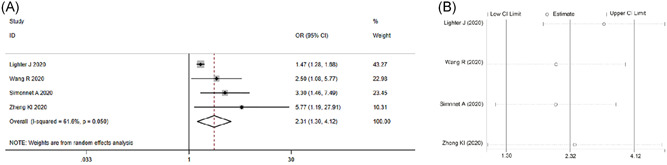
Meta‐analysis of the risk of obese patients to develop into severe COVID‐19. A, Forest plot. B, Sensitivity analysis
Considering the high heterogeneity among 4 included articles, we carried out a sensitivity analysis to verify their stability. The results indicate that no study changed the overall heterogeneity, which was also stable (Figure 3B).
As the number of included studies in this part was small, we abandoned attempts at subgroup analysis and meta‐regression.
3.4. Publication bias detection
We did not conduct publication bias detection for the meta‐analyses of BMI and disease severity, as the number of included articles was less than 10.
4. DISCUSSION
In this study, we searched the existing literature and combined the effect sizes through systematic review and meta‐analysis. Those with severe COVID‐19 may have a higher BMI, while obese patients were more likely to develop into severe conditions once infected with COVID‐19. These results indicate that obesity may exacerbate COVID‐19.
Lighter and colleagues have found that COVID‐19 patients younger than 60 were more likely to seek hospital or ICU admission when obese. Simonnet et al 10 found the proportion of COVID‐19 patients requiring mechanical ventilation to increase with BMI. As we were writing this article, Caussy et al 19 observed the same phenomenon as Simonnet. The results above add to our confidence on the role that obesity may play in COVID‐19 progression.
Obesity could lead to severe conditions of COVID‐19 in several possible ways, some of which have been published by scholars: (1) Obesity and the subsequent metabolic syndrome could cause damage to organs, which may turn into function failure when faced with such stress 20 , 21 ; (2) obesity is accompanied by increased expression of ACE2, which would bind to the virus S protein firmly and make the adipose tissue a portal for virus invasion, 22 making the lungs and heart vulnerable to virus attack 23 ; (3) obesity is accompanied by a state of overactivated inflammation and immune response, which may induce excessive inflammatory response and immune exhaustion in COVID‐19; (4) obese patients have increased abdominal pressure, limited chest expansion and movement, and insufficient respiratory compensatory function. In the case of lung infection, they are more likely to develop into respiratory failure. 24
Obesity, diabetes, and hypertension are all components of a metabolic syndrome and are the most common complications of COVID‐19, often occurring simultaneously in one patient. 25 A meta‐analysis showed that patients with diabetes and hypertension had a 2.61 and 2.84 risk of exacerbation, respectively. 26 The results of this study showed that obesity could increase the risk of exacerbation of COVID‐19 to 2.31. The OR value of hypertension is the largest from a numerical perspective, but considering the difference in patient population composition, we cannot conclude that hypertension is more likely to aggravate COVID‐19 than diabetes and obesity. The contribution of the above three factors to the exacerbation of COVID‐19 remains to be demonstrated by more large samples and high‐quality studies.
Certain limitations may exist in this study. Our meta‐analysis only contains nine studies. This may affect the reliability of our research results. Studies specific to associations between obesity and COVID‐19 are less common. We conducted this study based on limited literature to provide some updated evidence for future research. Another limitation may lie in the heterogeneity in the population considered obese in the studies analyzed. Different diagnostic criteria for obesity exist in the literature, which may be one of the sources of heterogeneity. Also, the included studies did not mention the detailed comorbidities of obese patients, such as diabetes and hypertension. This may confuse the role of obesity as an independent risk factor in COVID‐19.
As of our submission date, this is the only meta‐analysis focusing on the relationship between obesity and COVID‐19. We have searched and integrated all relevant information to confirm the hypothesis that obesity may aggravate COVID‐19. We hope clinical and basic researchers from all over the world can work together to elucidate the clinical significance and internal mechanism of this complex pathological process to lay a solid foundation for subsequent prevention and control.
5. CONCLUSION
We have conducted a systematic review and meta‐analysis and found that obesity could aggravate COVID‐19. Our results may have important implications for the clinical management and basic research on obesity and COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Yang J, Hu J, Zhu C. Obesity aggravates COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;93:257–261. 10.1002/jmv.26237
Contributor Information
Jun Yang, Email: yaju90@126.com.
Chunyan Zhu, Email: xijiangyue3013@163.com.
REFERENCES
- 1. Valle LF, Ruan D, Dang A, et al. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine. Berlin, Heidelberg: Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuin M, Rigatelli G, Zuliani G, Rigatelli A, Mazza A, Roncon L. Arterial hypertension and risk of death in patients with COVID‐19 infection: systematic review and meta‐analysis. J Infec. 2020;81(1):e84‐e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzola P, Rimoldi SM, Rossi P, et al. Aging in Italy: the need for new welfare strategies in an old country. Gerontologist. 2016;56(3):383‐390. [DOI] [PubMed] [Google Scholar]
- 4. Torres‐Gil FM, Suh EH, Angel J. Working across borders: the social and policy implications of aging in the Americas. J Cross Cult Gerontol. 2013;28(3):215‐222. [DOI] [PubMed] [Google Scholar]
- 5. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360. https://www.cdc.gov/nchs/products/index.htm [PubMed] [Google Scholar]
- 6. Marques A, Peralta M, Naia A, Loureiro N, de Matos MG. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Pub Health. 2018;28(2):295‐300. [DOI] [PubMed] [Google Scholar]
- 7. González‐Muniesa P, Mártinez‐González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- 8. Desai A, Sachdeva S, Parekh T, Desai R. COVID‐19 and cancer: lessons from a pooled meta‐analysis. JCO Global Oncol. 2020;6:557‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1193‐1199. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El‐Arabey AA, Abdalla M. Metformin and COVID‐19: A novel deal of an old drug [published online ahead of print, 2020 Apr 29]. J Med Virol. 2020. 10.1002/jmv.25958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiang TX, Liu JM, Xu F, et al. Analysis of clinical characteristics of 49 patients with coronavirus disease 2019 in Jiangxi. Chin J Respir Crit Care Med. 2020;19(2):154‐160. [Google Scholar]
- 13. Wang XF, Zhou ZQ, Yang HH, et al. Extrapulmonary organ damage and clinical significance in patients with coronavirus disease 2019. Zhejiang Med J. 2020;42(5):485‐488. [Google Scholar]
- 14. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng KI, Gao F, Wang XB, et al. Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV. Int J Cerebrovasc Dis. 2020;27(7):622‐666. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID‐19). J Intern Med. 2020;2:1‐11. [DOI] [PubMed] [Google Scholar]
- 18. Wang R, Xie LL, Du P, et al. Clinical characteristics of 96 hospitalized patients with coronavirus disease 2019. Chin J Respir Crit Care Med. 2020;19(2):144‐147. [Google Scholar]
- 19. Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Chin J Tuberculosis Respir Dis. 2020;43(3):209‐214. [DOI] [PubMed] [Google Scholar]
- 20. Caussy C, Wallet F, Laville M, et al. Obesity is associated with severe forms of COVID‐19. Obesity. 2020;28(7):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah D, Romero F, Guo Z, et al. Obesity‐induced endoplasmic reticulum stress causes lung endothelial dysfunction and promotes acute lung injury. Am J Respir Cell Mol Biol. 2017;57(2):204‐215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kassir R. Risk of COVID‐19 for patients with obesity. Obesity Rev. 2020;21(6):e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126:1456‐1474. 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid chronic diseases are strongly correlated with disease severity among COVID‐19 patients: a systematic review and meta‐analysis. Aging Dis. 2020;11(3):668‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]


