Abstract
Background
Patients with cancer have a higher risk of coronavirus disease 2019 (COVID‐19) than noncancer patients. The authors conducted a multicenter retrospective study to investigate the clinical manifestations and outcomes of patients with cancer who are diagnosed with COVID‐19.
Methods
The authors reviewed the medical records of hospitalized patients who were treated at 5 hospitals in Wuhan City, China, between January 5 and March 18, 2020. Clinical parameters relating to cancer history (type and treatment) and COVID‐19 were collected. The primary outcome was overall survival (OS). Secondary analyses were the association between clinical factors and severe COVID‐19 and OS.
Results
A total of 107 patients with cancer were diagnosed with COVID‐19, with a median age of 66 years (range, 37‐98 years). Lung (21 patients; 19.6%), gastrointestinal (20 patients; 18.7%), and genitourinary (20 patients; 18.7%) cancers were the most common cancer diagnoses. A total of 37 patients (34.6%) were receiving active anticancer treatment when diagnosed with COVID‐19, whereas 70 patients (65.4%) were on follow‐up. Overall, 52.3% of patients (56 patients) developed severe COVID‐19; this rate was found to be higher among patients receiving anticancer treatment than those on follow‐up (64.9% vs 45.7%), which corresponded to an inferior OS in the former subgroup of patients (hazard ratio, 3.365; 95% CI, 1.455‐7.782 [P = .005]). The detrimental effect of anticancer treatment on OS was found to be independent of exposure to systemic therapy (case fatality rate of 33.3% [systemic therapy] vs 43.8% [nonsystemic therapy]).
Conclusions
The results of the current study demonstrated that >50.0% of infected patients with cancer are susceptible to severe COVID‐19. This risk is aggravated by simultaneous anticancer treatment and portends for a worse survival, despite treatment for COVID‐19.
Keywords: anticancer treatment, cancer, case fatality rate, coronavirus disease 2019 (COVID‐19), systemic therapy
Short abstract
Patients with cancer who are receiving anticancer treatment have a 3‐fold higher risk of death from coronavirus disease 2019 (COVID‐19) than those on follow‐up. The authors report that this detrimental effect on survival appears to be independent of anticancer treatment modalities.
Introduction
There is an unprecedented outbreak of the novel coronavirus disease 2019 (COVID‐19) worldwide, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 , 3 , 4 This illness is characterized by fever, dyspnea, cough, and gastrointestinal symptoms of diarrhea, nausea, and vomiting. 5 As of April 22, 2020, the latest numbers indicated that there were >2,000,000 COVID‐19 cases worldwide, and >100,000 deaths had occurred. 6
We previously have shown that patients with cancer have a higher risk of contracting COVID‐19. 7 Compared with the community, the estimated incidence of COVID‐19 is approximately 2.3‐fold higher in this susceptible group of patients, and the risk is attributable to both active anticancer treatment and recurrent visits to the hospital without appropriate infection control measures in place. In addition, in our small case series, we observed that the median age of infected patients with cancer was older (>60 years) compared within the community, and these patients had a tendency to develop more severe illness. It also was suggested that among the different cancer types, patients with lung cancer who are aged >60 years are particularly at risk of COVID‐19. 7 Although it may appear to be intuitive that patients with an abnormal respiratory epithelium are likely to be more prone to rapid virus entry into the lungs, more data are needed to clarify some of these associations. Currently, several guidelines regarding the management of patients with cancer also have been proposed, but arguably, these are mostly consensus agreements, with little guidance derived from data regarding the outcomes of patients with cancer who are diagnosed with COVID‐19. 8 , 9 , 10 , 11 Examples of some pertinent questions include possible differences in the severity of COVID‐19 between patients with different cancer types and the implications of active anticancer treatment on the clinical presentation, severity, and treatment outcomes of COVID‐19 pneumonia.
To fill these gaps in knowledge, we conducted a multicenter retrospective study regarding the outcomes of patients with cancer who were diagnosed with COVID‐19. The primary aim of the current study was overall survival (OS). Secondary analyses included the reporting of clinical presentation and outcomes based on the different cancer types, and the association between mortality due to COVID‐19 and whether the patient was receiving ongoing active anticancer treatment (systemic therapy, local therapy, or a combination).
Materials and Methods
Patient Cohorts
The current study was a multicenter, retrospective, observational study of 5 hospitals (Zhongnan Hospital of Wuhan University, Leishenshan Hospital, the Fifth Hospital of Wuhan, the Seventh Hospital of Wuhan, and Wuhan Hankou Hospital) in Wuhan City, China. We reviewed the medical records of 3559 hospitalized patients with COVID‐19 who were treated at these institutions from January 5 to March 18, 2020. It is interesting to note that Wuhan Leishenshan hospital was 1 of 2 emergency makeshift hospitals that were constructed in the city to isolate and treat patients with COVID‐19 during the peak of the outbreak. Inclusion criteria included: 1) confirmation of a diagnosis of COVID‐19 based on the fifth edition criteria (see Supporting Information A, 5 ); 2) a prior confirmed histological and/or clinical diagnosis of cancer; and 3) available information regarding current and prior cancer treatments. There were no exclusion criteria. Th current study was approved by the ethics committee of the Zhongnan Hospital of Wuhan University (No. 2020041). Because anonymized, aggregated data were analyzed, a waiver of informed consent was approved by the institutional review board.
Diagnosis of COVID‐19
The diagnosis of COVID‐19 was made based on the fifth edition criteria, which were developed in response to the outbreak by the National Health Commission of China during the study period (Supporting Information A, section Supplementary Methods). Briefly, a diagnosis of COVID‐19 could be made if patients had a positive real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 and/or demonstrated characteristic findings of atypical pneumonia on computed tomography scan of the chest. 12 , 13 Typical computed tomography chest findings included bilateral pulmonary parenchymal ground‐glass and consolidative pulmonary opacities that were distributed in the peripheral zones of the lungs. 5 , 14
We further categorized patients into those with mild and those with severe COVID‐19. Patients were diagnosed as having severe COVID‐19 if they manifested any of the following clinical conditions: 1) tachypnea of ≥30 respiratory rate per minute; 2) oxygen saturation of ≤93% at room air; 3) arterial partial pressure of oxygen (PaO2) per fraction of inspired oxygen (FiO2) of ≤300 mm Hg; 4) respiratory failure requiring mechanical ventilation; 5) septic shock; and 6) multiorgan failure requiring care in the intensive care unit. 15
SARS‐Cov‐2 RT‐PCR Assay
All samples were processed at designated laboratories in compliance with the World Health Organization guidance. All samples were tested for SARS‐CoV‐2 by quantitative RT‐PCR using the kit recommended by the Centers for Disease Control and Prevention.
Statistical Analyses
We adhered to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) standards of reporting for the current cohort study. Frequencies and percentages were reported for categorical variables, whereas means (with standard deviations) and medians (with interquartile ranges [IQRs]) were used to describe quantitative data. The primary outcome was OS, which was defined as the time from the onset of symptoms to death from any cause. Secondary analyses included the percentage of patients with severe COVID‐19 and the association between clinical (cancer type, patient age, and comorbidities) and treatment parameters (status of anticancer treatment) and severe COVID‐19 and OS. Survival curves were illustrated using the Kaplan‐Meier method and compared using the log‐rank test. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated using the Cox proportional hazards model, and the proportional hazards assumption was tested with Schoenfeld residuals. OS was updated as of April 17, 2020.
Detailed information regarding demographics, smoking history (former vs current smokers), comorbidities (hypertension, diabetes, and cardiorespiratory conditions), signs and symptoms at onset, laboratory results (complete blood count, renal and liver panels, and inflammatory markers), and COVID‐19 treatment were collected. Patients also were classified based on whether they were receiving active anticancer treatment (including surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, endocrine therapy, and best supportive treatment) or were taking part in posttreatment follow‐up (defined as >1 month from the completion of treatment) at the time of diagnosis with COVID‐19. All statistical analyses were performed using SPSS statistical software (version 23.0; IBM Corporation, Armonk, New York). A 2‐sided P value <.05 was considered to be statistically significant. No correction for multiple testing was performed.
Results
Clinical Characteristics of Patients With Cancer Who Were Diagnosed With COVID‐19
Of 3559 patients, 107 patients were diagnosed with COVID‐19 and had a diagnosis of cancer (Supporting Information B); the breakdown of cases across the 5 institutions is summarized in Supporting Table 1 in Supporting Information A. The clinical characteristics of these patients were summarized in Table 1. The median age of the patients was 66 years (range, 36‐98 years). Approximately 56.1% of the patients (60 patients) were male and 43.9% (47 patients) were female. A total of 72 patients (67.3%) had a history of comorbidities, which included hypertension (52 patients; 48.6%), diabetes (22 patients; 20.6%), cardiac conditions (14 patients; 13.1%), and chronic obstructive pulmonary disease (5 patients; 4.7%); 9 patients had a positive smoking history.
Table 1.
Clinical Characteristics of 107 Patients With Cancer Who Were Diagnosed With COVID‐19
| Patient Characteristics | Total | Severity | |
|---|---|---|---|
| Mild | Severe | ||
| N = 107 | N = 51 | N = 56 | |
| Median age (range), y | 66 (36‐98) | 64 (37‐78) | 69 (36‐98) |
| Sex | |||
| Male | 60 (56.1%) | 23 (45.1%) | 37 (66.1%) |
| Female | 47 (43.9%) | 28 (54.9%) | 19 (33.9%) |
| Smoking history | |||
| Yes | 9 (8.4%) | 4 (7.8%) | 5 (8.9%) |
| No | 58 (54.2%) | 31 (60.8%) | 27 (48.2%) |
| Unknown | 40 (37.4%) | 16 (31.4%) | 24 (42.9%) |
| Cancer types | |||
| Lung | 21 (19.6%) | 10 (19.6%) | 11 (19.6%) |
| Gastrointestinal | 20 (18.7%) | 6 (11.8%) | 14 (25.0%) |
| Genitourinary | 20 (18.7%) | 9 (17.6%) | 11 (19.6%) |
| Head and neck | 17 (15.9%) | 10 (19.6%) | 7 (12.5%) |
| Breast | 10 (9.3%) | 4 (7.8%) | 6 (10.7%) |
| Hematological | 9 (8.4%) | 6 (11.8%) | 3 (5.4%) |
| Others (CNS/liver/pancreas) | 10 (9.3%) | 6 (11.8%) | 4 (7.1%) |
| Stage of disease a | |||
| I‐III | 84 (78.5%) | 44 (86.3%) | 40 (71.4%) |
| IV | 23 (21.5%) | 7 (13.7%) | 16 (28.6%) |
| Status of cancer care | |||
| On follow‐up | 70 (65.4%) | 38 (74.5%) | 32 (57.1%) |
| Receiving active treatment | 37 (34.6%) | 13 (25.5%) | 24 (42.9%) |
| Anticancer treatments | |||
| Chemotherapy/targeted therapy | 15 (14.0%) | 5 (9.8%) | 10 (17.9%) |
| Immunotherapy | 6 (5.6%) | 3 (5.9%) | 3 (5.4%) |
| Local treatment | 5 (4.7%) | 2 (3.9%) | 3 (5.4%) |
| Best supportive treatment | 11 (10.3%) | 3 (5.9%) | 8 (14.3%) |
| Comorbidities | |||
| Hypertension | 52 (48.6%) | 19 (37.3%) | 33 (58.9%) |
| Diabetes | 22 (20.6%) | 5 (9.8%) | 17 (30.4%) |
| CVD | 14 (13.1%) | 4 (7.8%) | 10 (17.9%) |
| COPD | 5 (4.7%) | — | 5 (8.9%) |
| Autoimmune diseases | 4 (3.7%) | 2 (3.9%) | 2 (3.6%) |
| Others | 15 (14.0%) | 7 (13.7%) | 8 (14.3%) |
Abbreviations: CNS, central nervous system; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; CVD, cardiovascular diseases.
Based on the Eighth Edition of Cancer Staging Manual by American Joint Committee on Cancer.
Types of Cancer and Status of Anticancer Treatment
Lung cancer was the most common diagnosis (21 patients; 19.6%), followed by gastrointestinal (20 patients; 18.7%), genitourinary (20 patients; 18.7%), and head and neck (17 patients; 15.9%) cancers (Table 1). A total of 84 patients (78.5%) had early‐stage cancers (stages I‐III, based on the Eighth Edition of Cancer Staging Manual by American Joint Committee on Cancer), and 23 patients (21.5%) had stage IV disease. Of these, only 37 patients (34.6%) were receiving active anticancer treatment at the time of their COVID‐19 diagnosis. Five patients had received or were continuing to receive local treatment (surgery and/or radiotherapy), and 21 patients were receiving systemic therapy either as monotherapy or in combination with local treatment. A total of 11 patients were receiving best supportive treatment alone (Table 1). Only 4 of 37 patients continued with their anticancer treatment after their diagnosis of COVID‐19; these individuals still were alive at the time of last follow‐up (April 17, 2020). Among the 70 patients on follow‐up, only 4 patients were within 1 year after treatment.
Clinical Manifestations
A total of 51 patients (47.7%) had mild COVID‐19, whereas 56 patients (52.3%) developed severe disease. The median time from the onset of symptoms to severe illness was 10 days (range, 1‐21 days). Fever (81 patients; 75.7%), cough (79 patients; 73.8%), fatigue (53 patients; 49.5%), and dyspnea (39 patients; 36.4%) were common symptoms at the onset of disease (Table 2). Less frequent symptoms included myalgia (20 patients; 18.7%) and diarrhea (15 patients; 14.0%). Patients receiving active anticancer treatment were more likely to have dyspnea (56.8% vs 25.7%) and myalgia (32.4% vs 11.4%) compared with those on follow‐up. It is important to note that more patients receiving active anticancer treatment manifested with severe COVID‐19 than those on follow‐up (24 of 37 patients [64.9%] vs 32 of 70 patients [45.7%]), which corresponded to more deaths (14 of 37 patients [37.8%] vs 9 of 70 patients [12.9%]) in the former group of patients (Table 2).
Table 2.
Clinical Symptoms, Treatment, and Outcomes of Patients With Cancer Who Were Diagnosed With COVID‐19
| Total | Follow‐Up | Receiving Anticancer Treatment | |
|---|---|---|---|
| N = 107 | N = 70 | N = 37 | |
| Signs and symptoms | |||
| Respiratory | |||
| Cough | 79 (73.8%) | 50 (71.4%) | 29 (78.4%) |
| Dyspnea | 39 (36.4%) | 18 (25.7%) | 21 (56.8%) |
| Gastrointestinal | |||
| Diarrhea | 15 (14.0%) | 12 (17.1%) | 3 (8.1%) |
| Constitutional symptoms | |||
| Fever | 81 (75.7%) | 54 (77.1%) | 27 (73.0%) |
| Fatigue | 53 (49.5%) | 37 (52.9%) | 16 (43.2%) |
| Myalgia | 20 (18.7%) | 8 (11.4%) | 12 (32.4%) |
| Treatment | |||
| Oxygen therapy | 91 (85.0%) | 60 (85.7%) | 31 (83.8%) |
| Mechanical ventilation | 18 (16.8%) | 7 (10.0%) | 11 (29.7%) |
| Antiviral therapy a | 99 (92.5%) | 65 (92.9%) | 34 (91.9%) |
| Steroid therapy | 39 (36.4%) | 31 (44.3%) | 8 (21.6%) |
| IVIG therapy | 22 (20.6%) | 17 (24.3%) | 5 (13.5%) |
| Complications of COVID‐19 | |||
| ARDS | 21 (19.6%) | 9 (12.9%) | 12 (32.4%) |
| Heart failure | 13 (12.1%) | 8 (11.4%) | 5 (13.5%) |
| Acute renal injury | 3 (2.8%) | 3 (4.3%) | — |
| Disease severity | |||
| Mild | 51 (47.7%) | 38 (54.3%) | 13 (35.1%) |
| Severe | 56 (52.3%) | 32 (45.7%) | 24 (64.9%) |
| Outcome status | |||
| Discharged | 84 (78.5%) | 61 (87.1%) | 23 (62.2%) |
| Died | 23 (21.5%) | 9 (12.9%) | 14 (37.8%) |
Abbreviations: ARDS, adult respiratory distress syndrome; COVID‐19, coronavirus disease 2019; IVIG, intravenous immunoglobulin.
Includes oseltamivir, umifenovir, interferon‐α, ribavirin, lopinavir, and chloroquine phosphate.
Table 3 shows the baseline hematological and biochemical parameters of the current study cohort. Lymphocytopenia (55 patients; 51.4%), elevated C‐reactive protein (62 patients; 57.9%), and procalcitonin (N = 52 patients; 48.6%) were the most common abnormalities among our patients with cancer. Next, we observed that patients receiving active anticancer treatment experienced more pronounced lymphopenia (median, 0.55×109/L [IQR, 0.29‐1.12×109/L] vs 1.05×109/L [IQR, 0.63‐1.55 ×109/L]), anemia (112.00 g/L [IQR, 96.00‐120.18 g/L] vs 122.50 g/L [IQR, 115.25‐129.75 g/L]), elevated C‐reactive protein (36.00 mg/L [IQR, 8.28‐103.75 mg/L] vs 27.40 mg/L [IQR, 2.43‐56.14 mg/L]), and procalcitonin (0.17ng/mL [IQR, 0.05‐0.77 ng/mL] vs 0.06 ng/mL [IQR, 0.04‐0.12 ng/mL]) compared with patients on follow‐up.
Table 3.
Laboratory Results of 107 Patients With Cancer Who Were Diagnosed With COVID‐19
| Laboratory Results a | Total | Follow‐Up | Receiving Anticancer Treatment |
|---|---|---|---|
| N = 107 | N = 70 | N = 37 | |
| Leukocytes, ×109/L | 5.20 (4.08‐6.74) | 5.20 (4.09‐6.62) | 5.19 (3.63‐7.63) |
| Neutrophils, ×109/L | 3.64 (2.53‐5.01) | 3.63 (2.80‐4.42) | 3.92 (2.44‐6.31) |
| Lymphocytes, ×109/L | 0.94 (0.49‐1.45) | 1.05 (0.63‐1.55) | 0.55 (0.29‐1.12) |
| Hemoglobin, g/L | 118.00 (106.10‐128.00) | 122.50 (115.25‐129.75) | 112.00 (96.00‐120.18) |
| Platelets, ×109/L | 187.50 (136.00‐234.75) | 203.00 (156.50‐244.75) | 153.00 (91.50‐211.00) |
| ALT, U/L | 23.00 (16.75‐39.00) | 28.00 (18.75‐39.50) | 20.00 (12.00‐37.75) |
| AST, U/L | 24.00 (18.00‐39.25) | 24.00 (18.00‐32.25) | 25.50 (16.50‐44.00) |
| Blood urea, mmol/L | 5.13 (3.95‐6.85) | 4.95 (3.95‐6.36) | 5.64 (3.85‐7.17) |
| Creatinine, umol/L | 66.80 (56.00‐83.13) | 69.45 (56.75‐82.63) | 63.95 (55.33‐87.95) |
| Albumin, g/L | 35.25 (32.03‐37.95) | 35.40 (33.00‐38.38) | 33.75 (29.95‐37.03) |
| C‐reactive protein, mg/L | 31.10 (3.00‐76.60) | 27.40 (2.43‐56.14) | 36.00 (8.28‐103.75) |
| Procalcitonin, ng/mL | 0.07 (0.05‐0.20) | 0.06 (0.04‐0.12) | 0.17 (0.05‐0.77) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019.
Data were expressed as median (IQR).
COVID‐19 Treatments and Outcomes
COVID‐19 treatments that were administered are summarized in Table 2. A total of 99 patients (92.5%) received antiviral therapy (oseltamivir, umifenovir, interferon‐α, ribavirin, lopinavir, and chloroquine phosphate), 39 patients (36.4%) received steroid therapy, and 22 patients (20.6%) received intravenous immunoglobulin (IVIG) therapy. A total of 91 patients (85.0%) required oxygen support; of these, 18 patients (16.8%) eventually were placed on mechanical ventilation. Patients receiving active anticancer treatment were more likely to require mechanical ventilation (11 of 37 patients [29.7%] vs 7 of 70 patients [10.0%]). However, a lower percentage of these patients received steroids (8 of 37 patients [21.6%] vs 31 of 70 patients [44.3%]) and IVIG (5 of 37 patients [13.5%] vs 17 of 70 patients [24.3%]).
In terms of the frequencies of severe COVID‐19 complications in the current study cohort, 21 patients (19.6%) developed acute respiratory distress syndrome, 13 patients (12.1%) developed heart failure, and 3 patients (2.8%) developed acute renal injury (Table 2). As of the time of last follow‐up on April 17, 2020, we recorded 23 (21.5%) COVID‐19‐related deaths, whereas 84 patients (78.5%) had been discharged. The case fatality rates for the overall study cohort, patients who were receiving active anticancer treatment, and those on follow‐up were 21.5% (23 of 107 patients), 37.8% (14 of 37 patients), and 12.9% (9 of 70 patients), respectively. We observed that patients receiving active anticancer treatment demonstrated a significantly worse prognosis than those who were on follow‐up (HR, 3.365; 95% CI, 1.455‐7.782 [P = .005]) (Fig. 1A). The disparity in OS also was observed between the 2 groups when patients receiving only best supportive treatment were excluded (HR, 3.117; 95% CI, 1.236‐7.859 [P = .016]) (Fig. 1B). The median duration from the onset of symptoms to recovery and death was 31 days (range, 8‐53 days) and 20 days (range, 6‐45 days), respectively.
Figure 1.
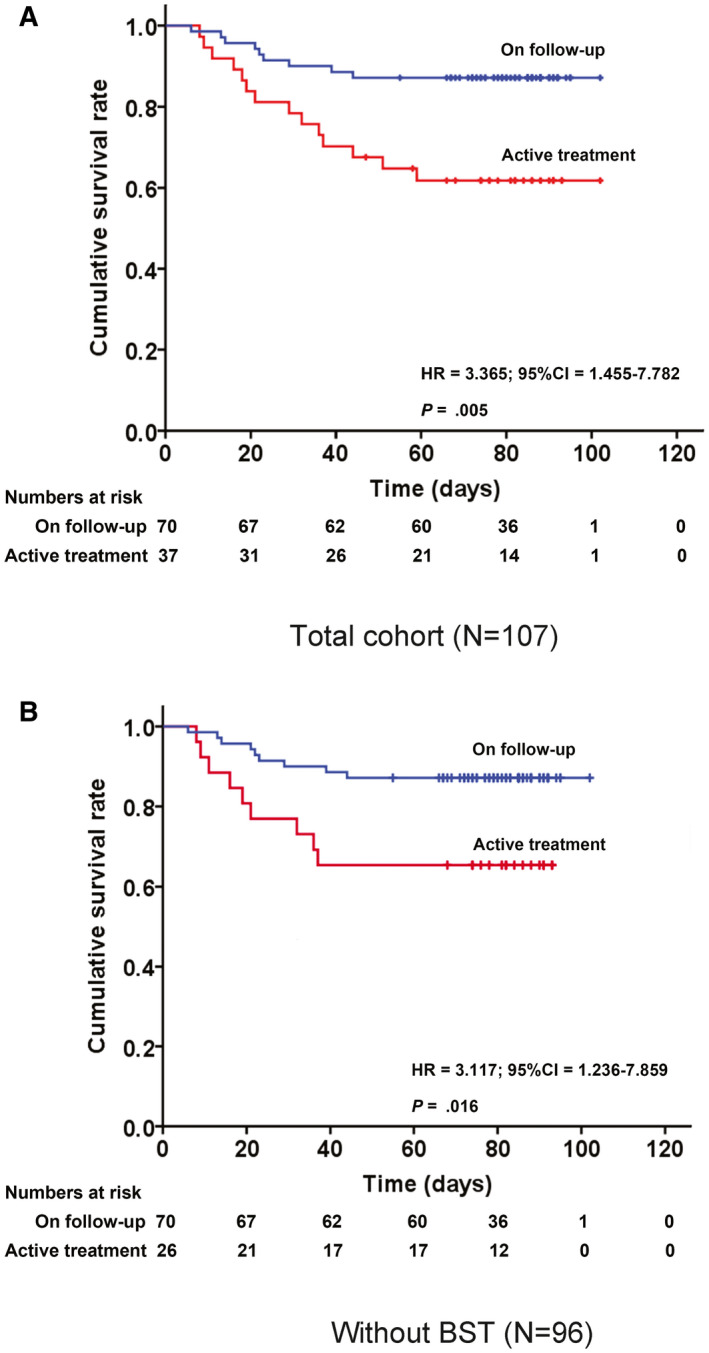
Overall survival of patients with cancer who were diagnosed with coronavirus disease 2019 (COVID‐19) who received anticancer treatment and those who were on follow‐up. (A) Kaplan‐Meier plot for the entire cohort of 107 patients and (B) Kaplan‐Meier plot with the 11 patients who were receiving best supportive treatment (BST) excluded. HR indicates hazard ratio.
Association Between Cancer Type and Anticancer Treatment Status and COVID‐19 Severity and Mortality
We further investigated potential associations between clinical and treatment parameters and the likelihood of severe COVID‐19 and death. We observed that patients with gastrointestinal cancers were most likely to experience severe illness (Fig. 2A), and this corresponded to 35% of deaths (7 of 20 patients) for this cancer type (Fig. 2B). Age (adjusted HR, 1.053; 95% CI, 1.007‐1.101 [P = .023]) and receiving active anticancer treatment (adjusted HR, 3.56; 95% CI, 1.53‐8.277 [P = .003]) were found to be significant predictors of OS after a diagnosis of COVID‐19 (Table 4). However, the effect of active anticancer treatment on COVID‐19 severity and OS appeared to be consistent regardless of whether the patient received systemic therapy (Fig. 2C,D) (Table 4) (see Supporting Table 2 in Supporting Information A).
Figure 2.
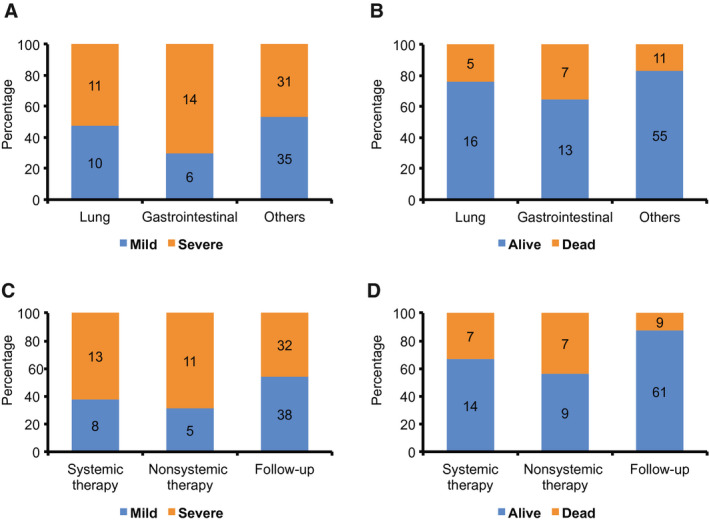
Comparison of coronavirus disease 2019 (COVID‐19) severity and mortality for different cancer types and cancer treatment modalities and status. (A and B) Comparison between patients with lung, gastrointestinal, and other cancers. (C and D) Comparison between patients receiving systemic therapy (chemotherapy, targeted therapy, immunotherapy, and endocrine therapy), those receiving nonsystemic therapy (surgery, radiotherapy, and best supportive treatment), and those patients on follow‐up.
Table 4.
Univariable and Multivariable Tests of Association Between Clinical and Treatment Parameters and Overall Survival In 107 Patients With Cancer Who Were Diagnosed With COVID‐19
| Variables | Univariable Analysis | Multivariable Analysis (23 Events) | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (continuous) | 1.046 (1.004‐1.090) | .031 | 1.053 (1.007‐1.101) | .023 |
| Comorbidity (yes vs no; reference) | 0.922 (0.399‐2.131) | .850 | ||
| Cancer types | ||||
| Lung (reference) | ||||
| Gastrointestinal | 1.885 (0.506‐7.021) | .345 | ||
| Genitourinary | 2.783 (0.815‐9.510) | .103 | ||
| Head and neck | 1.087 (0.243‐4.857) | .913 | ||
| Other | 1.776 (0.444‐7.104) | .417 | ||
| Receiving anticancer treatment (yes vs no; reference) | 3.365 (1.455‐7.782) | .005 | 3.560 (1.532‐8.277) | .003 |
| Follow‐up (reference) | ||||
| Chemotherapy/targeted therapy | 2.878 (0.964‐8.594) | .058 | ||
| Immunotherapy | 3.075 (0.664‐14.248) | .151 | ||
| Local treatment | 4.487 (0.969‐20.800) | .055 | ||
| Best supportive treatment | 3.769 (1.261‐11.259) | .018 | ||
Abbreviations: 95% CI, 95% confidence interval; COVID‐19, coronavirus disease 2019; HR, hazard ratio.
Bold type highlighted the results with statistical significance (P value <.05).
Discussion
In this multicenter retrospective study, we have reported our experience regarding the management and outcomes of patients with cancer who also contracted SARS‐CoV‐2 infection. Herein, we have reported the clinical symptoms and trajectory of COVID‐19 in 107 patients with different cancer types. It is important to note that the results of the current study demonstrated that this susceptible patient subgroup is at risk of developing severe COVID‐19 (52.3% overall) and the risk of death is significantly associated with a recent or concurrent exposure to anticancer treatment (HR >3.0). At the time of last follow‐up on April 17, 2020, approximately 21.5% of patients in the current study cohort had died of COVID‐19 or COVID‐19‐related complications; this finding is consistent with those of other case series in patients with cancer, but is in contrast to the rates that had been reported in the community (3.0% in noncancer patients as reported by Guan et al 16 ) (see Supporting Table 3 in Supporting Information A). This is a crucial observation, especially when the oncology community remains uncertain regarding the implications of initiating or continuing anticancer treatment for patients with cancer who are diagnosed with COVID‐19 during this pandemic, given the scarcity of evidence. Prior to this study, a smaller case series by Zhang et al among 28 patients with cancer also demonstrated an interaction between recent exposure to anticancer treatment within 14 days of a COVID‐19 diagnosis and severity of the illness. 17 Taken together, the current study data support the belief that anticancer treatment should be best avoided, if possible, in patients with cancer who unfortunately contract SARS‐CoV‐2 infection. In addition, for asymptomatic or noninfected individuals who are undergoing anticancer treatment, the results from the current study strengthen the argument for tight infection control measures to prevent virus transmission to patients within the hospital or ambulatory treatment facility.
We made several interesting observations in the current study cohort. Foremost, we previously had reported a preliminary observation demonstrating that older patients (ie, those aged >60 years) who are diagnosed with lung cancer are at a higher risk of COVID‐19. 7 In this larger study, we again observed that patients with lung cancer constituted a high percentage of COVID‐19 cases (19.6%) among patients with different cancer types, although the percentages were comparable to those of patients with gastrointestinal, genitourinary, and head and neck cancers. Although these patients may be at risk of contracting COVID‐19, we did not observe that they necessarily had a higher incidence of severe illness compared with patients without lung cancer (Fig. 2A), which would suggest that synchronous or metachronous lung cancers do not influence the pathogenetic mechanisms underpinning severe COVID‐19. 18 , 19 Next, although patients with cancer were likely to experience severe COVID‐19, their clinical presentation and laboratory parameters were comparable to the trends that were reported in the general community. 5 , 16 Rather, in the current study cohort of 107 patients, we observed that active anticancer treatment within 1 month of a diagnosis of COVID‐19 was associated with profound lymphopenia (median of 0.55 vs 1.05 for those on follow‐up) and markedly elevated inflammatory markers of C‐reactive protein (36.00 vs 27.40) and procalcitonin (0.17 vs 0.06). Among the constellation of COVID‐19 symptoms, myalgia and dyspnea were more frequent among patients receiving anticancer treatment compared with those who were on surveillance. It is interesting to note that we found that fewer of these patients in the former subgroup of patients received COVID‐19 therapies; approximately 21.6% received steroid therapy and 13.5% received IVIG compared with 44.3% and 24.3%, respectively, in the latter patient subgroup. Although we could not determine the potential reasons underpinning such a trend, it must be rationalized that the efficacy of these therapies in the treatment of patients with COVID‐19 remains questionable. Thus, we judged that this observation may not have biased the association between cancer treatment status and OS.
We next explored the interactions between the different types of anticancer therapies and the likelihood of death from COVID‐19 in the current study cohort. It is interesting to note that we observed that patients who were receiving systemic therapy (including chemotherapy, targeted therapy, immunotherapy, and endocrine therapy) were not more susceptible to severe illness and death than those who received local therapy or best supportive treatment (Fig. 2C,D). In addition, we questioned whether anticancer agents such as immune checkpoint blockade inhibitors potentially could worsen the trajectory of COVID‐19 pneumonia. 20 , 21 Nonetheless, we did not observe any disparate effects on the severity and case fatality rate between patients receiving conventional systemic agents and immunotherapy (see Supporting Table 2 in Supporting Information A), although the current analysis was limited by the smaller numbers in each treatment group. On this note, larger cohort studies with the aggregation of multiple data sets will help to provide more granular insights in this regard, as well as examine the interactions of all the potential clinical confounders such as comorbidities, cancer types, and their specific treatments, and COVID‐19 treatment on the outcomes of patients with cancer.
Conclusions
The results of the current study demonstrated the clinical characteristics and outcomes of COVID‐19 in a large cohort of 107 patients with different cancer types, and suggested a high incidence of severe illness and case fatality rates compared with the community population. These adverse outcomes were observed among patients who either were receiving anticancer treatment or were on surveillance, but the risk of death was significantly worse in the former patient subgroup. Based on this and other studies, it therefore is imperative to consider the deferment of anticancer treatment, if possible, in patients with cancer who unfortunately are diagnosed with COVID‐19. In the same vein, we reiterate that tight infection control measures are crucial to prevent the risk of virus transmission to patients who are receiving ongoing anticancer treatment.
Funding Support
The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the article; and the decision to submit the article for publication.
Conflict of Interest Disclosures
Conghua Xie is supported by the Health Commission of Hubei Province Scientific Research Project (WJ2019H002); the Health Commission of Hubei Province Medical Leading Talent Project; Fundamental Research Funds for the Central Universities (2042018kf1037 and 2042019kf0329); the Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018005); and the Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (znpy2017049 and znpy2018070). Melvin L. K. Chua has received grants and personal fees from Ferring Singapore; has acted as a paid member of the advisory board for Janssen, Astellas, Merck, and Illumina; has acted as a paid member of the advisory board and received hardware for research from Varian; has received nonfinancial support (provision of an artificial intelligence contouring work station for research) from PAVmed Inc; and has received nonfinancial support from MedLever Inc and Decipher Biosciences for work performed outside of the current study. Dr. Chua also is supported by the National Medical Research Council Clinician‐scientist award (NMRC/CSA/0027/2018). Xiaokun Shen is the founder and Chief Executive Officer of Convalife (Shanghai) Company Ltd and has an equity interest. Xiaokun Shen was supported in part by the National Science and Technology Major Projects for Major New Drugs Innovation and Development (2019ZX09301010) and Pudong New Area Science and Technology Development Foundation (PKX2019‐S08). The other authors made no disclosures.
Author Contributions
Concept and design: Hongyan Zhang, Yahua Zhong, Xinghuan Wang, Melvin L. K. Chua, and Conghua Xie. Acquisition, analysis, and interpretation of the data: All authors. Statistical analysis: Hongyan Zhang, Linwei Wang, Qiuji Wu, and Melvin L. K. Chua. Administrative, technical, or material support: Yahua Zhong, Melvin L. K. Chua, and Conghua Xie. Study supervision: Yahua Zhong, Xinghuan Wang, Melvin L. K. Chua, and Conghua Xie. Drafting of the article: Hongyan Zhang, Linwei Wang, Qiuji Wu, Melvin L. K. Chua, and Conghua Xie. Final approval of the article: All authors. Melvin L. K. Chua and Conghua Xie had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Supplementary Material
Supplementary Material
Zhang H, Wang L, Chen Y, Wu Q, Chen G, Shen X, Wang Q, Yan Y, Yu Y, Zhong Y, Wang X, Chua MLK, Xie C. Outcomes of novel coronavirus disease 2019 (COVID‐19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020:126:4023‐4031. 10.1002/cncr.33042
The first 6 authors are co‐first authors.
The last 4 authors are co‐senior authors.
We thank all patients and frontline health care workers who are involved in the coronavirus disease 2019 (COVID‐19) pandemic.
Contributor Information
Yahua Zhong, Email: chxie_65@whu.edu.cn, Email: melvin.chua.l.k@singhealth.com.sg, Email: wangxinghuan@whu.edu.cn, Email: doctorzyh73@163.com.
Xinghuan Wang, Email: wangxinghuan@whu.edu.cn.
Melvin L. K. Chua, Email: melvin.chua.l.k@singhealth.com.sg.
Conghua Xie, Email: chxie_65@whu.edu.cn.
Data Availability Statement
The data regarding the baseline patient information, survival outcomes, and detailed treatment information will be deposited in the Research Data Deposit public platform (www.researchdata.org.cn). The other data supporting the findings of the current study are available within the article and its supporting information files and from the corresponding authors upon request.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong JEL, Leo YS, Tan CC. COVID‐19 in Singapore‐current experience: critical global issues that require attention and action. JAMA. Published online February 20, 2020. doi:10.1001/jama.2020.2467 [DOI] [PubMed] [Google Scholar]
- 4. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Coronavirus disease 2019 (COVID‐19) Situation Report‐63. Accessed March 24, 2020. www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200323‐sitrep‐63‐covid‐19.pdf?sfvrsn=d97cb6dd_2
- 7. Yu J, Ouyang W, Chua MLK, et al. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. Published online March 25, 2020. doi:10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yahalom J, Dabaja BS, Ricardi U, et al. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID‐19 pandemic. Blood. 2020;135:1829‐1832. doi:10.1182/blood.2020006028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk‐adapted head and neck cancer radiotherapy during the COVID‐19 pandemic: an ASTRO‐ESTRO consensus statement. Int J Radiat Oncol Biol Phys. Published online April 14, 2020. doi:10.1016/j.ijrobp.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fakhry N, Schultz P, Moriniere S, et al; French Society of Otorhinolaryngology, Head and Neck Surgery (SFORL) ; French Society of Head and Neck Carcinology (SFCCF) . French consensus on management of head and neck cancer surgery during COVID‐19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:159‐160. doi:10.1016/j.anorl.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: an international collaborative group. Oncologist. Published online April 3, 2020. doi:10.1634/theoncologist.2020‐0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Information for laboratories about coronavirus (COVID‐19) . Accessed February 5, 2020. www.cdc.gov/coronavirus/2019‐ncov/lab/rt‐pcr‐detection‐instructions.html
- 14. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Central People’s Government of the People's Republic of China . Accessed February 8, 2020, in Chinese. http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf
- 16. Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for COVID‐19 . Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi:10.1183/13993003.00547‐2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. Published online March 26, 2020. doi:10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. J Infect. 2020;80:607‐613. doi:10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kattan J, Kattan C, Assi T. Do checkpoint inhibitors compromise the cancer patients' immunity and increase the vulnerability to COVID‐19 infection? Immunotherapy. 2020;12:351‐354. doi:10.2217/imt‐2020‐0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bersanelli M. Controversies about COVID‐19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
The data regarding the baseline patient information, survival outcomes, and detailed treatment information will be deposited in the Research Data Deposit public platform (www.researchdata.org.cn). The other data supporting the findings of the current study are available within the article and its supporting information files and from the corresponding authors upon request.


