The clinical picture of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2)/coronavirus disease 2019 (COVID‐19)‐related acute respiratory syndrome is often associated with a coagulopathy. 1 An elevated D‐dimer has been linked with an unfavourable prognosis in patients with COVID‐19. In a recent cohort study, 71% of patients who died matched the International Society of Thrombosis and Hemostasis (ISTH) criteria for disseminated intravascular coagulation (DIC) 2 , while this percentage was only 0·6% in patients who survived. 3 In two recent studies, the authors described the presence of a hypercoagulability state in COVID‐19 affected patients, in the absence however of pathognomonic signs of DIC. 4 , 5 Thus, the nature of this coagulopathy is not fully understood. Clinical evidence suggests that this COVID‐19‐related coagulopathy is associated with an increased risk of both venous and arterial thrombotic events. 6 , 7 The management of these thromboembolic complications is based on the use of heparin in the absence of contraindications (active bleeding and a platelet count of <30 × 109/l). 8 However, the efficacy of heparin remains to be validated. The benefit/risk of using heparin, as well as the timing of starting anti‐coagulants and at which dose, is controversial. 9 , 10 We carried out an observational study to investigate different coagulation parameters, in particular the thrombin generation assay (TGA), in critically ill patients with COVID‐19 and to correlate these results with different doses of low‐molecular‐weight heparin (LMWH) administered to these patients. The study was approved by the Ethics Committee of the Sapienza University of Rome (no. 109/2020). The TGA is a global coagulation test that provides a direct assessment of plasma coagulability. 11 The following TGA parameters were evaluated: T‐Lag, time that follows the addition of the trigger until the initiation of thrombin generation; time to peak (tt‐Peak), time to the highest thrombin concentration; thrombin peak (Peak), the highest thrombin concentration; endogenous thrombin potential (ETP), area under the curve, total amount of thrombin generation. Between April and May 2020, a consecutive series of 27 patients with COVID‐19 admitted to the Intensive Care Unit (ICU) of the Sapienza University Hospital in Rome were included in the study; 17 (63%) were males and 10 (37%) were females. The mean (range) age was 66 (38–85) years. At the time of the sample collection, patients were swab‐culture positive for COVID‐19, were affected by acute respiratory failure without an active and diagnosed thromboembolic event. All patients were intubated and mechanically ventilated. In all, 14 patients (51·9%) were treated with low‐dose LMWH (100 iu/kg/day) and 13 (48·1%) with high‐dose LMWH (100 iu/kg/twice daily). After the initial administration of LMWH at a dose of 100 iu/kg, based on the growing evidence of potential thrombotic events we decided to increase the prophylactic dose up to 100 iu/kg twice daily. 12 This is why we observed two differently treated populations. The median (range) time from the first LMWH administration and the blood sample collection was 5 (2–21) days; laboratory assays were evaluated on a single occasion and all blood samples were collected just before starting the LMWH administration. We observed an increase in the mean D‐dimer values to 4686·07 ng/ml [normal value (n.v.) <550 ng/ml], as well as of factor VIII (FVIII), von Willebrand factor antigen level (VWF:Ag) and VWF ristocetin co‐factor (VWF:RCo): 209% (n.v. 58–130%), 319·54% (n.v. 50–160%) and 310·60% (n.v. 52–124%) respectively (Table I). Increased levels of FVIII are a potent sign of hypercoagulability and increased levels of VWF:Ag and VWF:RCo are indicative of an endothelial derangement. These altered coagulation parameters observed in patients with COVID‐19 could be related to an active response due to a marked alveolar inflammatory cell infiltrate with a consequent systemic cytokine storm. On the contrary, we found laboratory parameters compatible with a diagnosis of DIC only in a few patients: a prolonged prothrombin time (PT) was observed in 29·6% of patients, a prolonged activated partial thromboplastin time (aPTT) in 14·8%, a reduced fibrinogen in 29·6%, a reduced antithrombin (AT) in 37% and a decreased platelet count in 7·4% (Table I). We cannot exclude that these results may be associated with the heparin administration, which was ongoing in all patients from at least 2 days prior to the blood collection. With regard to the TGA, we observed overall increased mean values of the T‐Lag (7·70 min; n.v. <4·3 min), Peak (122·22 nM; n.v. <106·2 nM) and tt‐Peak (13·38 min; n.v. <9·8 min); the mean ETP was within the normal range (953·51 nM min; n.v. <984·12 nM min). All parameters were influenced by LMWH administration in a dose‐dependent manner. In fact, we observed an increased mean value of the T‐Lag and tt‐Peak, and a decrease in the mean value of Peak and ETP in the group of patients on high‐dose LMWH compared to the data observed in patients on low‐dose LMWH (Table I, Fig 1). ETP was the only parameter that was significantly increased (P = 0·046), probably because ETP is influenced more by LMWH dosing. A thrombotic complication occurred in three of the 27 patients (11%): two pulmonary embolisms and one acute myocardial ischaemia (MI). All three patients were on low‐dose LMWH. The patient with the MI was previously on high‐dose LMWH, but because of a tracheostomy site bleed, he was switched to the low‐dose regimen: 3 days later the patient experienced the MI. The low rate of thromboembolic complications observed in our present patients is probably due to the fact that all patients were given LMWH prophylaxis as soon as they arrived in the ICU and that half of them were on high‐dose LMWH. Moreover, in our present patients with COVID‐19 the use of heparin did not result in a higher risk of bleeding complications: we observed only one haemorrhagic event. In conclusion, our present results suggest that critically ill patients with COVID‐19 develop a state of hypercoagulability that is also present during the administration of LMWH. Different doses of LMWH have an influence on the laboratory results, especially for the total amount of thrombin generation, with a significant reduction only in patients receiving high‐dose heparin. In our experience, the use of a higher dose of LMWH, as thromboembolic prophylaxis, reduced the incidence of thrombotic complications without an increase in bleeding events. Randomised clinical trials are required to conclusively define the efficacy and safety of different doses of LMWH in patients with severe COVID‐19 infection. Moreover, further studies on the utility of changes in thrombin generation parameters in predicting risk of thromboembolism in patients severely affected with COVID‐19 are required.
Table I.
Coagulation parameters in the 27 patients with COVID‐19 (mean values).
| Coagulative tests (normal range) | Mean value (range) (n = 27) | HD patients, mean value (n = 14) | LD patients, mean value (n = 13) | P (HD vs. LD) |
|---|---|---|---|---|
| PT ratio (0·92–1·18) | 1·12 (0·9–1·64) | 1·18 | 1·06 | 0·11 |
| aPTT ratio (0·81–1·20) | 1·14 (0·81–3·06) | 1·21 | 1·07 | 0·31 |
| Fibrinogen, mg/l (196–440) | 383·44 (62–663) | 365 | 404 | 0·9 |
| AT, % (80–120) | 88·88 (67·7–120) | 90 | 87 | 0·64 |
| D‐dimer, ng/ml (<5500) | 4686·07 (465–35 782) | 4409 | 4985 | 0·48 |
| Platelets, × 109/l (100–450) | 214 (51–378) | 213 | 216 | 0·9 |
| FVIII, % (58–130) | 209·07 (108·6–392·9) | 214 | 203 | 0·66 |
| VWF:Ag,% (50–160) | 319·54 (158·4–557·1) | 352 | 284 | 0·1 |
| VWF:RCo, % (52–124) | 310·60 (143·5–600·6) | 357 | 261 | 0·12 |
| PC, % (70–115) | 114·35 (72·5–149·8) | 114 | 115 | 0·96 |
| PS, % (64–124) | 71·14 (35·9–98·2) | 73 | 69 | 0·61 |
| T‐Lag, min (≤4·3) | 7·70 (3–32·17) | 8·9 | 6·5 | 0·32 |
| tt‐Peak, min (≤9·8) | 13·38 (5·17–49·67) | 15·4 | 11·2 | 0·81 |
| Peak, nM (≤106·2) | 122·22 (5·31–268·48) | 98·1 | 148·4 | 0·69 |
| ETP, nM min (≤984·12) | 953·51 (1–2357·21) | 705·19 | 1222·52 | 0·01 |
aPTT, activated partial thromboplastin time; AT, antithrombin; ETP, endogenous thrombin potential; FVIII, factor VIII; HD, high‐dose LMWH; LD, Low‐dose LMWH; PC, protein C; Peak, thrombin peak (highest thrombin concentration); PS, protein S; PT, prothrombin time; T‐Lag, time that follows the addition of the trigger until the initiation of thrombin generation; tt‐Peak, time to the highest thrombin concentration; VWF:Ag, von Willebrand factor antigen level; VWF:RCo, VWF ristocetin co‐factor.
The bolded values are the abnormal findings of our study.
Fig. 1.
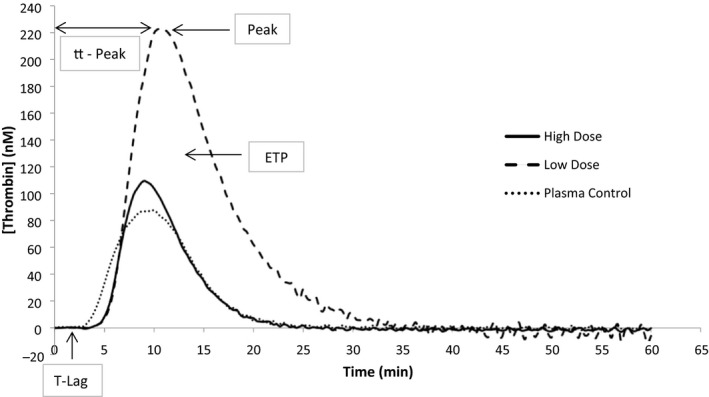
Thrombin generation curves in two patients (one patient on high‐ and one on low‐dose LMWH) and normal plasma control.
Conflict of interest
The authors have no competing interests.
Author contributions
Antonio Chistolini, Franco Ruberto, Fabio M. Pulcinelli, Francesco Pugliese conceived and designed the study. Francesco Alessandri, Cristina Santoro collected the clinical and epidemiological data and analysed the results. Francesco Barone, Maria Cristina Puzzolo performed tests and analysed the data. Giancarlo Ceccarelli, Massimo Mancone, Maria L. De Luca analysed clinical, epidemiological data and performed statistical analyses. Franco Ruberto, Antonio Chistolini, Francesco Alessandri, Cristina Santoro wrote the manuscript. Robin Foà, Massimo Mancone, Domenico Alvaro, Francesco Pugliese reviewed the manuscript. All the authors revised the final version.
Supporting information
Table SI. Coagulation parameters in the 27 patients with COVID‐19 (median values).
Data S1. Supplementary methods.
Acknowledgements
Work partly supported by Associazione Italiana Ricerca sul Cancro (AIRC), Special 5 × 1000 Program Metastases (21198), Milan (Italy) to Franco Ruberto.
Policlinico Umberto I COVID‐19 Group: Alida Albante, Francesco Alessandri, Domenico Alvaro, Guido Antonelli, Fabio Araimo‐Morselli, Daniela Auricchio, Erminia Baldacci, Francesco Barone, Federico Bilotta, Matteo Brisciani, Katia Bruno, Alessandro Cappannoli, Vincenzo Cardinale, Giancarlo Ceccarelli, Paola Celli, Antonio Chistolini, Stella Consolo, Claudia Croce, Beatrice Crocitti, Mariaignazia Curreli, Gabriella D'Ettorre, De Lauri Daniela, Francesco De Lazzaro, Maria Lucia De Luca, Francesco Fedele, Antonietta Ferretti, Gioacchino Galardo, Lorena Giannetti, Stefano Ianni, Carmela Imperiale, Viviana Maestrini, Eugenia Magnanimi, Federica Maldarelli, Massimo Mancone, Maurizio Martelli, Sabina Martelli, Claudio Mastroianni, Teresa Messina, Sandra Olivieri, Agnese Pallotta, Elisa Pattelli, Filippo Pecorari, Serena Perrella, Mario Piazzolla, Monica Portieri, Francesco Pugliese, Fabio Pulcinelli, Fabiola Ratini, Claudia Ricci, Franco Ruberto, Pietro Santopietro, Cristina Santoro, Alessandra Serrao, Guglielmo Tellan, Luca Titi, Paolo Tordiglione, Antonella Tosi, Fausto Trigilia.
Contributor Information
Antonio Chistolini, Email: antonio.chistolini@uniroma1.it.
the Policlinico Umberto I COVID‐19 Group:
Alida Albante, Francesco Alessandri, Domenico Alvaro, Guido Antonelli, Fabio Araimo‐Morselli, Daniela Auricchio, Erminia Baldacci, Francesco Barone, Federico Bilotta, Matteo Brisciani, Katia Bruno, Alessandro Cappannoli, Vincenzo Cardinale, Giancarlo Ceccarelli, Paola Celli, Antonio Chistolini, Stella Consolo, Claudia Croce, Beatrice Crocitti, Mariaignazia Curreli, Gabriella D'Ettorre, De Lauri Daniela, Francesco De Lazzaro, Maria Lucia De Luca, Francesco Fedele, Antonietta Ferretti, Gioacchino Galardo, Lorena Giannetti, Stefano Ianni, Carmela Imperiale, Viviana Maestrini, Eugenia Magnanimi, Federica Maldarelli, Massimo Mancone, Maurizio Martelli, Sabina Martelli, Claudio Mastroianni, Teresa Messina, Sandra Olivieri, Agnese Pallotta, Elisa Pattelli, Filippo Pecorari, Serena Perrella, Mario Piazzolla, Monica Portieri, Francesco Pugliese, Fabio Pulcinelli, Fabiola Ratini, Claudia Ricci, Franco Ruberto, Pietro Santopietro, Cristina Santoro, Alessandra Serrao, Guglielmo Tellan, Luca Titi, Paolo Tordiglione, Antonella Tosi, and Fausto Trigilia
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis. 2006;17:445–51. [DOI] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID‐19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42. 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin‐Loeches I, Browne P, et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18:1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Napolitano M, Saccullo G, Marietta M, Carpenedo M, Castaman G, Cerchiara E, et al. Platelet cut‐off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: an expert consensus. Blood Transfus. 2019;17:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thachil J, Tang N, Gando S, Falanga A, Levi M, Clark C, et al. Type and dose of heparin in Covid‐19. J Thromb Haemost. 2020. [Online ahead of print]. DOI: 10.1111/jth.14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, et al. COVID‐19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020;18:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707. [DOI] [PubMed] [Google Scholar]
- 12. Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID‐19. Intensive Care Med. 2020. [Online ahead of print]. DOI: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Coagulation parameters in the 27 patients with COVID‐19 (median values).
Data S1. Supplementary methods.


