Abbreviations
- COVID‐19
coronavirus disease 2019
- IS
immunosuppressive
- LT
liver transplantation
- NPS
nasal‐pharyngeal swab
- PLT
pediatric liver transplantation
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
TO THE EDITOR:
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) disease, coronavirus disease 2019 (COVID‐19), represents an unprecedented public health issue for the general population and for patients with underlying chronic conditions.( 1 ) Compared with adults, children seem to have a milder course of the disease, with very few requiring medical attention.( 2 )
Reports of critical disease in adults and children with cancer have raised concerns about the risk of severe COVID‐19 course in patients with immune system impairment, including recipients of solid organ transplants on longterm immune suppression.( 3 )
Because severe COVID‐19 represents an immune‐inflammatory overresponse to SARS‐CoV‐2, immune suppression might not represent an issue or even be protective against lung injury.( 4 ) Recent reports by our team are reassuring on the basis of direct experience and the lack of any evidence that immunosuppression was an additional risk factor for the disease in previous coronavirus epidemics.( 5 , 6 )
Thousands of children are living worldwide with a liver transplantation (LT), but no reports have been released about the impact of COVID‐19 in this population, especially due to the difficulties in obtaining clinical information during the pandemic.
As the most affected region in a country where true infections certainly exceed those detected by orders of magnitude, Lombardy can be considered one of the areas with the highest COVID‐19 prevalence worldwide.( 7 ) The Hospital Papa Giovanni XXIII in Bergamo, which is located in the epicenter of the outbreak, is also the largest Italian pediatric liver transplantation (PLT) center.
To overcome the limitations in patients’ access to health care during the outbreak, we designed a telephone survey aiming to collect information on the health status and the exposure risk during the SARS‐CoV‐2 pandemic of children followed at our PLT center and residing in a high‐prevalence area in northern Italy.
Data records of all patients who underwent LT between 0 and 17 years of age, as a part of the Bergamo program from 1997 to 2019, and residing in Lombardy between January 2020 and April 2020 were selected. Between April 6 and 17, 2020, a 18‐question phone interview to the patients or patients’ caregivers was carried out to address the following: demographic features, patient medical history and immunosuppressive (IS) treatment, history of contacts with suspected or confirmed COVID‐19 cases, occurrence of symptoms suggestive for COVID‐19, referral to a hospital for any reason, testing for SARS‐CoV‐2 infection, and the presence of suspected or confirmed COVID‐19 among family members or relatives (Supporting Information).
Suspected cases of COVID‐19 were defined by at least 1 of the following 2 conditions:
An episode of acute respiratory tract infection (sudden onset of at least 1 of the following: cough, fever, or shortness of breath).
Close contact with a confirmed or highly probable patient with SARS‐CoV‐2 infection.( 1 )
Confirmed patients with COVID‐19 were defined by a positive nasal‐pharyngeal swab (NPS) for SARS‐CoV‐2 nucleic acid testing using real‐time reverse‐transcriptase polymerase chain reaction assay.( 1 ) The severity of illness was classified as in previous studies.( 1 , 2 )
We based the epidemiological assumptions on a recently published SARS‐CoV‐2 prevalence model that estimated that 13.3% of the Lombardy population has been infected (corresponding to approximately 1,338,056 patients).( 8 ) To minimize the proportion of undetected SARS‐CoV‐2 in patients within the pediatric age range given the lack of severity of symptoms, we selected population data from the Veneto region with the highest number of diagnostic tests performed in relation to the population.( 9 ) Assuming that the age distribution of true patients in our region is close to that of the Veneto region (10.3% of the patients in the age group 0‐19 years), considering local census data,( 10 ) we calculated that approximately 137,820 patients with COVID‐19 occurred in Lombardy in patients aged 0‐29 years. With the previously mentioned assumptions, the estimated prevalence in our population should have been as high as 4873 (95% confidence interval, 4847‐4899) COVID‐19 patients per 100,000 inhabitants by the first week of April 2020.
Out of 519 patients, 364 residing outside the region of Lombardy during the study period were excluded (Fig. 1). A total of 155 patients (150 Italian patients residing in Lombardy, 5 cross‐border patients temporarily residing in Bergamo) were included in the study (male:female = 85:70; mean age, 13.6 years; interquartile range, 12.4‐14.8 years).
FIG. 1.
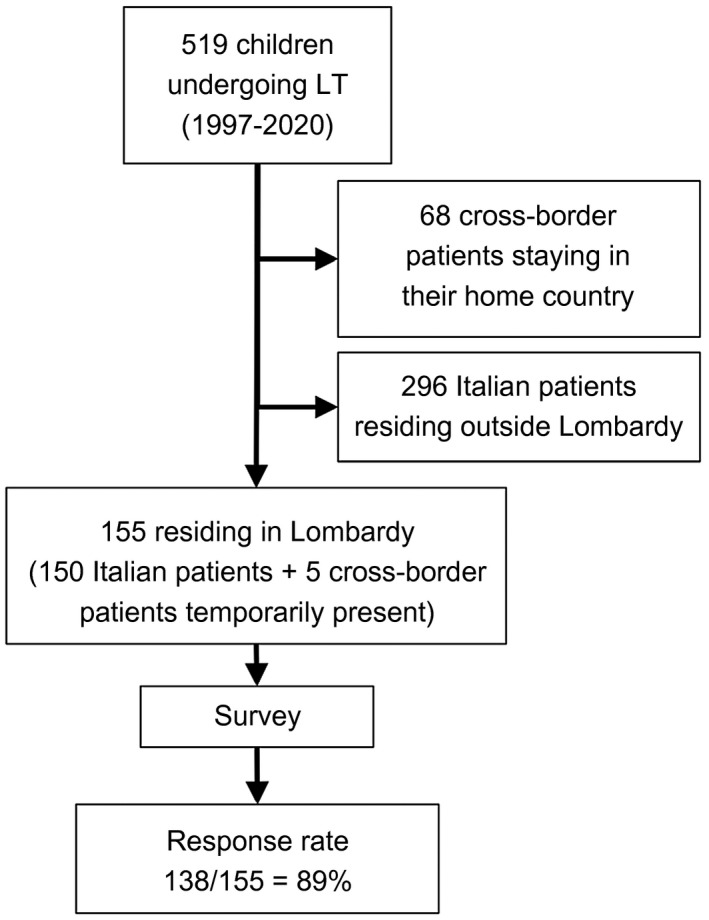
A flowchart of the patient cohort.
Questionnaires were administered by telephone to the caregivers or to the patients themselves in 138 patients (response rate = 89%). A total of 156 PLTs were performed in these 138 patients at a mean age of 3.5 years (interquartile range, 2.8‐4.3 years), in 3 patients combined with kidney transplants, and in 1 patient in the context of a multivisceral transplant. Biliary atresia was the primary disease leading to PLT in 55% of patients. The level of completeness of each question was high (overall percentage of received answers = 99.6%). All patients (100%) were on IS treatment (data on 138 patients, percentage of received answers 100%). Patients’ characteristics are displayed in Table 1.
TABLE 1.
Characteristics of 138 PLT Recipients Exposed to SARS‐CoV‐2 During the Outbreak in Northern Italy
| Number of Patients | Value (n = 138) |
|---|---|
| Sex, female | 60 (43.5) |
| Age at PLT, years | 1.5 (0.3‐17.9) |
| Age at survey, years | 14.9 (1.2‐34.8) |
| 0‐9 years | 44 (31.8) |
| 10‐19 years | 75 (54.3) |
| 20‐29 years | 17 (12.3) |
| ≥30 years | 2 (1.4) |
| Type of graft | |
| Whole liver | 21 (15.2) |
| Segments 2 + 3 | 100 (72.5) |
| Segments 1 and 4‐8 | 10 (7.2) |
| Other | 7 (5.1) |
| Patients on IS treatment | 138 (100) |
| Tacrolimus monotherapy | 103 (74.6) |
| Tacrolimus + prednisone | 13 (9.4) |
| Tacrolimus + mycophenolate | 12 (8.6) |
| Tacrolimus + prednisone + mycophenolate | 5 (3.6) |
| Cyclosporine monotherapy | 3 (2.2) |
| Tacrolimus + azathioprine | 1 (0.7) |
| Sirolimus monotherapy | 1 (0.7) |
| Contact with suspected case of COVID‐19 | 13 (9.4) |
| Contact with confirmed case of COVID‐19 | 5 (3.6) |
| Suspected COVID‐19 as per symptoms | 44 (32) |
| Fever | 24 (17.4) |
| Cough | 24 (17.4) |
| Fatigue | 5 (3.6) |
| Sore throat | 5 (3.6) |
| Diarrhea and/or vomiting | 5 (3.6) |
| Loss of appetite | 1 (0.7) |
| Anosmia | 1 (0.7) |
| Dysgeusia | 1 (0.7) |
| Shortness of breath | 1 (0.7) |
| Myalgia | 1 (0.7) |
| Suspected COVID‐19 as per contact with confirmed case | 2 (1.4) |
| Total suspected COVID‐19 infections | 48 (34.7) |
| Confirmed COVID‐19 infections | 0 (0) |
| Discontinuation of IS therapy | 0 (0) |
| Outcome | |
| Survived | 138 (100) |
| Hospitalized for suspected COVID‐19 | 0 (0) |
| Died | 0 (0) |
Data are given as n (%) or median (range).
Of 138 LT patients, 5 (4%) had close contact with a confirmed patient with COVID‐19, and for 3 LT patients, the COVID‐19–infected person was a parent. None of the patients developed symptoms suggestive of COVID‐19, nor required oxygen administration or hospitalization for reasons related to COVID‐19. An NPS was performed in 3 of these patients and yielded negative results.
Another 13 (9%) patients had contact with a suspected patient with COVID‐19, and the person was a household member for 5 patients. Eight patients (61%) developed symptoms compatible with COVID‐19, which were fever and cough in 7 (54%), fatigue in 2 (15%), and anosmia, dysgeusia, and loss of appetite in 1 patient. None of these patients received a diagnostic NPS or chest imaging for suspected pneumonia because none required medical attention for the respiratory illness.
There were 44 (32%) patients who developed respiratory symptoms including fever (17%), cough (17%), sore throat (4%), fatigue (4%), vomiting (4%), and diarrhea (3%). Two patients presented gastrointestinal symptoms in the absence of respiratory illness. Overall, about one‐third of the surveyed patients had symptoms compatible with COVID‐19 in the study period, occurring 38 ± 26 days before the interview. Only 1 patient was tested by NPS, which was negative for SARS‐CoV‐2.
None of the patients had respiratory failure, developed pneumonia, needed oxygen administration, nor required hospital admission. No patient reduced or withdrew IS treatment. A total of 39 (85%) patients had a full recovery by the time of the interview; the others were at home in good general conditions.
No confirmed case of COVID‐19 occurred in our surveyed population.
With the previously mentioned epidemiological assumptions, ie, the estimated prevalence in our population = 4873 SARS‐CoV‐2 patients/100,000, 7 infected patients were expected in our population. Considering patients with SARS‐CoV‐2 infection, all patients reporting respiratory symptoms, and the 2 with histories of close contact with confirmed COVID‐19 patients, a total number of 48 patients can be defined as suspected.
Although an epidemiological criterion for suspicion of COVID‐19 was present in 18 (13%), only 4 developed mild symptoms after being in contact with a confirmed or suspected patient with COVID‐19 and, interestingly, none of the 5 household contacts of confirmed COVID‐19 developed symptoms or was found positive for SARS‐CoV‐2 on diagnostic NPS. This raises questions about whether children are less susceptible to SARS‐CoV‐2 infection and not only to its more severe clinical implications.
On the other hand, about one‐third of our patients reported mild or moderate respiratory symptoms possibly compatible with SARS‐CoV‐2 infection in the study period. Because of the reduced diagnostic capability and limited diagnostic policies, NPS was not performed in such patients. However, so‐defined suspected COVID‐19 patients in the group of children and young adults receiving LT exceeded the estimated number of infections in the age‐matched general population by 7‐fold (34,782 versus 4873 patients per 100,000). The most likely hypothesis to explain this difference is that the vast majority of mild respiratory syndromes are due to other etiologies than SARS‐CoV‐2. In line with this, 18 children admitted to our pediatric hepatology ward for all causes in the study period had a negative SARS‐CoV‐2 screening NPS. Conversely, the calculated number of infections in the young general population could be still underestimated, as an effect of the poor diagnostic effort at the community level.( 11 )
The main finding of this research is that our young LT patients did not experience any severe respiratory infections, despite residing in one of the earliest‐ and hardest‐hit areas worldwide, where the first patient infections likely date long before any distancing or isolation measures were in place. This raises the question whether immunosuppression could be irrelevant or even protective against SARS‐CoV‐2 infection and its complications, which are mainly driven by a well‐documented proinflammatory state.
Similar results were obtained in children receiving anticancer chemotherapy and in those taking immune‐suppressant medications for inflammatory bowel disease.( 6 , 12 )
In conclusion, children, even when immunosuppressed, remain at low risk of severe or complicated COVID‐19 disease. In this emerging public health context, there are no reasons to delay or interrupt oncological treatments or withdraw immune suppression, to postpone lifesaving treatments in LT patients, or to suspend transplant programs as an a priori preventative measure.
Further research will be needed to understand the actual incidence of SARS‐CoV‐2 infections in at‐risk children and in the general pediatric population.
Supporting information
Supplementary Material
Lorenzo D'Antiga consults for Mirum Pharmaceuticals, Vivet Therapeutics, and Spark Therapeutics and advises for Alexion Pharmaceuticals.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 2. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID‐19 among children in China. Pediatrics 2020;145:320200702. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID‐19–infected cancer patients: a retrospective case study in three hospitals within Wuhan. Ann Oncol 2020;31:894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal 2020;10:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020;26:832‐834. [DOI] [PubMed] [Google Scholar]
- 6. Norsa L, Indriolo A, Sansotta N, Cosimo P, Greco S, D’Antiga L. Uneventful course in Patients with inflammatory bowel disease during the severe acute respiratory syndrome coronavirus 2 outbreak in northern Italy. Gastroenterology 2020;S0016‐5085(20)30445‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flaxman S, Mishra S, Gandy A, Unwin H, Coupland H, Mellan T, et al. Report 13: estimating the number of infections and the impact of non‐pharmaceutical interventions on COVID‐19 in 11 European countries. London, UK: Imperial College London. http://spiral.imperial.ac.uk/handle/10044/1/77731. Accessed April 27, 2020. [Google Scholar]
- 8. Signorelli C, Scognamiglio T, Odone A. COVID‐19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed 2020;91:175‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ministero della Salute . Nuovo coronavirus. http://www.salute.gov.it/nuovocoronavirus. Accessed April 27, 2020. [Google Scholar]
- 10. Indicatori demografici. http://dati.istat.it/Index.aspx?QueryId=18462. Accessed April 27, 2020. [Google Scholar]
- 11. Goumenou M, Sarigiannis D, Tsatsakis A, Anesti O, Docea AO, Petrakis D, et al. COVID‐19 in northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review). Mol Med Rep 2020;22:20‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hrusak O, Kalina T, Wolf J, Balduzzi A, Provenzi M, Rizzari C, et al. Flash survey on severe acute respiratory syndrome coronavirus‐2 infections in paediatric patients on anticancer treatment. Eur J Cancer 2020;132:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


