Abstract
Coronavirus disease‐19 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is one of the most contagious diseases in human history that has already affected millions of lives worldwide. To date, no vaccines or effective therapeutics have been discovered yet that may successfully treat COVID‐19 patients or contain the transmission of the virus. Scientific communities across the globe responded rapidly and have been working relentlessly to develop drugs and vaccines, which may require considerable time. In this uncertainty, repurposing the existing antiviral drugs could be the best strategy to speed up the discovery of effective therapeutics against SARS‐CoV‐2. Moreover, drug repurposing may leave some vital information on druggable targets that could be capitalized in target‐based drug discovery. Information on possible drug targets and the progress on therapeutic and vaccine development also needs to be updated. In this review, we revisited the druggable targets that may hold promise in the development of the anti‐SARS‐CoV‐2 agent. Progresses on the development of potential therapeutics and vaccines that are under the preclinical studies and clinical trials have been highlighted. We anticipate that this review will provide valuable information that would help to accelerate the development of therapeutics and vaccines against SARS‐CoV‐2 infection.
Keywords: clinical trial, COVID‐19, drug repurposing, drug targets, therapeutics, vaccines
1. INTRODUCTION
COVID‐19 that was first surfaced in China has already emerged as a global pandemic with a total of 10,421,869 cases, including 508,422 deaths, as of June 30, 2020 (worldometers.info/coronavirus/). COVID‐19 and the similar previous outbreaks such as SARS and Middle East Respiratory Syndrome (MERS) share common signs and symptoms, such as sore throat, persistent high fever, pneumonia with common pathologies, such as immune dysfunction, and multi‐organ failure (Andersen, Rambaut, Lipkin, Holmes, & Garry, 2020; Forster, Forster, Renfrew, & Forster, 2020). Like other coronaviruses, SARS‐CoV‐2 possesses several structural and nonstructural proteins that are implicated in crucial cell functions (Ashour, Elkhatib, Rahman, & Elshabrawy, 2020). Of the structural proteins, spike glycoproteins facilitate the attachment of the virus to their host cells. The helicase, main protease (Mpro), papain‐like protease (PLpro), RNA dependent RNA polymerase (RdRp) are among the nonstructural proteins that are critical for viral replication (Forster et al., 2020; Siu et al., 2008). With their significant roles in the viral life cycle, these proteins present promising pharmacological targets for developing potential therapeutics against SARS‐CoV‐2 infection.
Target‐based drug discovery (TDD) is one of the most successful and efficient strategies to find the remedy of any disease. An estimated 70% of the first‐class drugs approved by the United States Food and Drug Administration in between 1999 to 2013 were based on TDD (Croston, 2017; Eder, Sedrani, & Wiesmann, 2014). TDD is not only restricted to the discovery of small molecules as drug targets but also extends to protein biologics, genetic materials, and antibodies. The advantages of target‐based strategies over phenotypic approaches include high throughput, convenient, and less expensive (Croston, 2017; Zheng, Thorne, & McKew, 2013). Molecular pharmacology, biochemistry, genomics, computational modeling, structural biology, system biology, and mutational analysis are usually employed to carry out the TDD for the complete understanding of any drug candidate and also ensuring the effective therapeutics for upcoming days (Croston, 2017).
Although research communities have responded rapidly, still there is no vaccine or effective therapeutics for COVID‐19 (Harrison, 2020). Some of the existing antiviral drugs from other viral diseases, including Remdesivir, are being considered as repurposing medicine to treat COVID‐19 patients, which resulted in mixed outcomes (Gautret et al., 2020; Molina et al., 2020). It is, therefore, crucial to focus on alternative rational strategies for the development of urgent therapeutics to control the ongoing pandemic. Developing new therapeutic drugs and vaccines requires a proper understanding of the disease, the immune response pattern after infection, and the pathogenesis of the virus. To accelerate this effort, we present a scoping review summarizing the potential drug targets and therapeutics under trials by critically analyzing all relevant published literature on this field. This review would provide concrete evidence concerning the drug targets, previous use of the drugs, and early findings of possible drug mechanisms. Moreover, our review would provide insightful evidence for future SARS‐CoV‐2 research and might support researchers to discover a potential remedy.
2. STRUCTURE AND LIFE CYCLE OF BETACORONA VIRUS
SARS‐CoV‐2 is a member of the betacoronavirus genera and possesses 16 nonstructural proteins (NSP) and four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The N protein clamps the Ribonucleic acid (RNA) genome, whereas the S, E, and M proteins together shape the viral envelope, and the glycoprotein spikes render coronaviruses to form their unique crown‐like appearance (Figure 1a; Anthony et al., 2017; Ashour et al., 2020). In addition to the knowledge on the structural makeup, a critical understanding of the life cycle and pathogenesis of SARS‐CoV, in general, is essential to discover potential vaccines and therapeutics to prevent and treat SARS‐CoV and SARS‐like coronavirus (SL‐CoV), including SARS‐CoV‐2 infections in the future. Crucial steps of SARS‐CoV life cycle are, therefore, illustrated in Figure 1b and also highlighted below:
Receptor recognition is the first step of virus entry into the host cell that occurs by the recognition of the receptor by the spike protein of the virus. First, the receptor‐binding domain (RBD) of S protein attaches with the host cell receptor, angiotensin‐converting enzyme 2 (ACE2). S protein is then proteolytically cleaved by a type II transmembrane protease (TMPRSS2) resulting into two subunits called S1 and S2 where S2 is crucial for membrane fusion and virus entry (Glowacka et al., 2011; Holmes, 2003; Li, 2015; Li et al., 2006; Wan, Shang, Graham, Baric, & Li, 2020; Wong, Li, Moore, Choe, & Farzan, 2004). Splitting of the S protein by proteases is essential for the viral entry to the host cells in a pH‐dependent manner (Simmons et al., 2004; Yang & Shen, 2020).
After uncoating, CoVs then release the RNA (5′ methylated cap and a 3′ polyadenylated tail) into the host cell cytoplasm for replication (Fehr & Perlman, 2015; Kim et al., 2020; Li, 2015).
Genomic RNA of CoV acts as an mRNA for translation of the replicase polyproteins 1a (pp1a) and 1ab (pp1ab) using the translation machinery of the host cell. Then the polyprotein is split into effector proteins by viral proteinases (3CLpro and PLpro), resulting in a number of NSPs including RdRp and helicase that form the replicase–transcriptase complex (Báez‐Santos, St John, & Mesecar, 2015; Fehr & Perlman, 2015; Gorbalenya et al., 2020; Kim et al., 2020; Ratia et al., 2006; Sola, Almazán, Zúñiga, & Enjuanes, 2015).
RdRp and helicase localize to double‐membrane vesicles and produce subgenomic RNA, from which other proteins such as structural proteins are produced by translation. At the same time, the full‐length positive‐strand RNA is synthesized as genomic RNA (Fehr & Perlman, 2015; C. J. Gordon et al., 2020; Sola et al., 2015; Kim et al., 2020).
NSP 3, 4, and 6 are responsible for anchoring the coronavirus replication and transcription complex through recruitment of intracellular Endoplasmic Reticulum (ER) membrane to form double‐membrane vesicles (Fehr & Perlman, 2015; Sola et al., 2015; Stertz et al., 2007).
Viral nucleocapsids are assembled with genomic RNA and N protein in the cytoplasm, followed by budding into the lumen of the ERGIC (Endoplasmic Reticulum–Golgi Intermediate Compartment; Sola et al., 2015; Stertz et al., 2007).
The virions are then released from the cell through exocytosis (Sola et al., 2015; Stertz et al., 2007).
FIGURE 1.
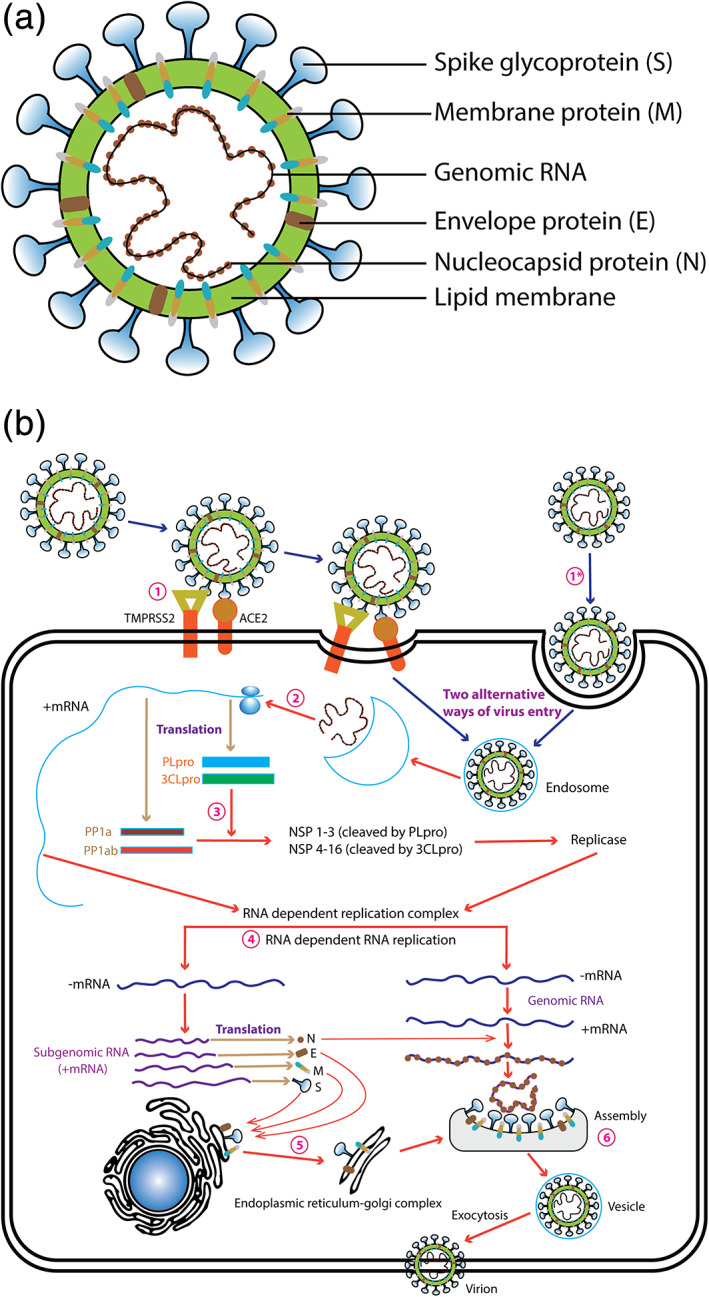
(a) A schematic diagram of the SARS‐CoV‐2 (modified from Du et al., 2009). S, Spike protein; E, envelope protein; single‐stranded positive‐sense viral RNA, and M, membrane protein are depicted. (b) A schematic diagram of the SARS‐CoV‐2 life cycle in a host cell (modified from Du et al., 2009). (1) Virus entry into the host cell via ACE2 or endocytosis, (2) Release of RNA from endosome, (3) Proteolytic cleavage of polyprotein, (4) synthesis of genomic and subgenomic RNA, (5) structural protein synthesis and maturation, and (6) Assembly of viral particles and mature viral particle. 3CLpro, chymotrypsin‐like protease; ACE2, Angiotensin‐converting enzyme 2; E, Envelope protein; M, membrane protein; N, nucleocapsid; PLpro, papain‐like protease; S, Spike protein; TMPRSS2, Transmembrane protease, serine 2
3. POTENTIAL DRUGGABLE TARGETS FOR SARS‐COV‐2 INFECTION
To find a potential therapy for SARS‐CoV‐2, a concrete understanding of potential drug targets is essential. Herein, we revisited a comprehensive list of SARS‐CoV‐2‐directed targets (Mpro, PLpro, RdRp, spike protein, helicase, envelop protein) and host‐directed targets (ACE2, TMPRSS2, and immune system) that might hold promise for the development of potential therapy against SARS‐CoV‐2 infections (Figure 2).
FIGURE 2.

A schematic diagram indicating the potential drug targets and drugs on viral replication processes. (1) ACE2 and spike complex inhibitors: Azithromycin and Chloroquine, interrupt in ACE2 and S protein complex formation; (2) TMPRSS2 inhibitor: Camostat Mesylate blocks cleavage and activation of the S protein; (3) Targeting endosomes: chloroquine and its derivatives increase pH and inhibit viral RNA release from endosomes; (4) Targeting proteases: ASC09F, Oseltamivir, Ritonavir, Oseltamivir, Oseltamivir, Lopinavir inhibit proteases thereby inhibit viral replication; (5) Targeting RdRp: Favipiravir, Remdesivir, and Ribavirin block RdRp and cause premature termination of RNA synthesis; (6) Targeting Spike: Monoclonal antibody, Arbidol, and convalescent plasma block spike protein which results into binding interruption with ACE2; (7) Targeting host immune system: activated host immune system can block virus propagation as well as recover body from the adverse effects posed by viruses
3.1. SARS‐CoV‐2‐directed targets
3.1.1. Non‐structural protein targets
Main protease (Mpro)
Two large overlapping replicase polyproteins (pp1a and pp1ab), encoded by two linked ribosomal frameshift, are hydrolyzed by an extensive proteolytic action of viral proteases (Fehr & Perlman, 2015; Graham, Sparks, Eckerle, Sims, & Denison, 2008; Pillaiyar, Manickam, Namasivayam, Hayashi, & Jung, 2016). One of the crucial viral proteases is the Mpro, also known as 3C‐like proteinase or NSP5, which is a homodimeric cysteine protease belonging to the coronavirus polyprotein group (Fan et al., 2004). Automatic cleavage of polyproteins leads to the generation of Mpro, which cleaves the large polyprotein 1ab at 11 cleavage sites to generate NSPs. The identification of cleaving sequence at most sites is Leu‐Gln↓(Ser, Ala, Gly) to release NSP4–NSP16 (Pillaiyar et al., 2016; Yang et al., 2005; Zhang, Penninger, Li, Zhong, & Slutsky, 2020). Mpro directly mediates the maturation of NSPs, which is critical for the initiation of the SARS‐CoV‐2 replication cycle and could, therefore, be a potential druggable target (Zhang, Wu, & Zhang, 2020). For instance, pyridone‐containing α‐ketoamides inhibited the Mpro activity showing early promise for the development of anti‐viral drugs (Zhang et al., 2020). Moreover, more than a hundred PDB structures of SARS‐CoV‐2 Mpro have already been deposited at the RCSB database (https://www.rcsb.org/) and more are being added regularly. Here, most of the structures were elucidated by Cryo‐electron microscopy, NMR or X‐ray crystallography, or even the high‐throughput network‐based inhibitor screening or direct ligand‐based study (Jin et al., 2020; Vuong et al., 2020). While ligand‐based inhibitors screening leads to the determination of complexed crystal structure of SARS‐CoV‐2 Mpro, this approach aids in the structure‐assisted drug design and high‐throughput screening for potential inhibitors (Jin et al., 2020). The inhibitors targeting SARS‐CoV‐2 Mpro are virus‐specific and, therefore, will not interfere with host cell proteases (Bzówka et al., 2020).
Papain like protease
Proteolytic enzymes play an essential role in viral replication, and hence could be the target of choice for the development of effective therapy against viral infections. PLpro, also termed as NSP 3, had been extensively studied for coronaviruses (Báez‐Santos et al., 2015; Lei et al., 2014; Ratia et al., 2006) and the efficacy of PLpro inhibitors such as chloroquine and formoterol have been investigated recently for the treatment of COVID‐19 (Rimanshee, Amit, Vishal, & Mukesh, 2020; Wu et al., 2020). PLpro cleaves the N‐terminal part of the polyproteins producing mature NSP1, NSP2, and NSP3, a process that is crucial for viral replication. The cleavage specificity of PLpro corresponds to the pattern (R/K)L(R/K)GG↓X. Moreover, the enzyme acts as deubiquitinase that removes (poly) ubiquitin units from proteins tagged with them (Barretto et al., 2005; Lindner et al., 2005). The deubiquitinase activity of PLpro interferes nuclear import of interferon‐regulatory factor 3 and the phosphorylation, thereby preventing the production of type‐I interferons, such as IFN‐α and IFN‐β by the infected host cell and also helps in peptide substrate recognition (Barretto et al., 2005; Clementz et al., 2010; Devaraj et al., 2007; Frieman, Ratia, Johnston, Mesecar, & Baric, 2009; Ratia et al., 2006). Additionally, PLpro clears ubiquitin and interferon‐sensitive gene 15 from host‐cell proteins, a mechanism that aids coronaviruses to evade host innate immune responses. Finally, the PLpro has been reported to interfere with the nuclear factor κB pathway, which further assists SARS‐CoV to counteract the innate immune response of the infected cells (Clementz et al., 2010). Therefore, pharmacological targeting of PLpro by developing PLpro inhibitors might constitute a potential therapeutic strategy that would be advantageous in not only preventing viral replication but also ensuring the normal regulation of signal networks in the infected cells.
RNA dependent RNA polymerase
RdRp, also termed as NSP 12, is a pivotal enzyme of RNA viruses, including coronavirus, that catalyzes the replication of RNA from an RNA template, probably with the assistance of NSP7 and NSP8 as co‐factors (Guo, Huang, Lin, & Lv, 2020; Kirchdoerfer & Ward, 2019; Subissi et al., 2014; Venkataraman, Prasad, & Selvarajan, 2018). A protein complex consisting of NSP 7, NSP 8, and NSP 12 of SARS‐CoV‐2 is related to that of SARS‐CoV with a root‐mean‐square deviation value of 0.82 for 1,078 Cɑ atoms (Gao et al., 2020). RdRp protein of SARS‐CoV‐2 and SARS‐CoV share 96% amino acid sequence identity and 82% sequence identity at their genomic RNA level (Morse, Lalonde, Xu, & Liu, 2020). The RdRp of SARS‐CoV‐2 contains a large and deep groove as an active site for the polymerization of RNA. Residues that show variations between the SARS‐CoV‐2 and SARS‐CoV RdRps are mostly distal to this active site. This high sequence conservation and structural homogeneity of RdRp in coronaviruses suggest that nucleotide analogs developed for the SARS‐CoV RdRp are likely to be effective against the SARS‐CoV‐2 RdRp (Morse et al., 2020). Previously published literature provides convincing evidence that RdRp could be a druggable target for the development of potent therapy against SARS‐CoV‐2 infection (Kandeel, Ibrahim, Fayez, & Al‐Nazawi, 2020; Lung et al., 2020), which includes nucleotide analog targeting RdRp (Deval, Jin, Chuang, & Kao, 2017; Gordon et al., 2020; Stephen & Lin, 2018; Tchesnokov, Feng, Porter, & Götte, 2019). Remdesivir (GS‐5734), a prodrug and adenosine analog, can be incorporated into nascent viral RNA, and subsequently inhibit the RdRp (Siegel et al., 2017). Furthermore, Remdesivir showed high considerable efficacy in controlling SARS‐CoV‐2 infection in a recent in vitro study (Wang et al., 2020). β‐D‐N4‐hydroxycytidine, a ribonucleoside analog, also inhibits SARS‐CoV‐2 replication as evidenced by reducing viral genomic RNA (Sheahan et al., 2020).
Helicase
SARS‐CoV helicase (NSP13) is one of the 16 NSPs generated after the proteolytic cleavage of two large polyproteins (pp1a and pp1ab). This helicase, being one of the components of the replicase–transcriptase complex, plays significant roles in the life cycle of SARS‐CoV (Marra et al., 2003; Subissi et al., 2014), which constitutes the basis of targeting this enzyme for the development of potential antiviral agent. Helicases are motor proteins that unwind double‐stranded nucleic acids into two single‐strands during replication (Adedeji et al., 2014; Shum & Tanner, 2008). Moreover, NSP13 N‐terminus carries 26 cysteines where 14 positions are highly conserved and assumed to possess a binuclear Zn2+‐binding cluster (Seybert et al., 2005). Importantly, an antiviral compound Bananin, which is an adamantane derivative, has been reported to inhibit helicase activities, thereby preventing the replication of SARS‐CoV (; Tanner et al., 2003) and can also be evaluated against SARS‐CoV‐2.
3.1.2. Structural protein target
Spike glycoprotein
The spike protein (S), giving a crown‐like appearance to virion surface, render diverse molecular actions that usually mediates the coronavirus entry into the specific host (Belouzard, Chu, & Whittaker, 2009). The spike protein of SARS‐CoV and SARS‐CoV‐2 shares ∼76% amino acid identity; however, SARS‐CoV‐2 S protein binds 10 times more strongly than SARS‐CoV S protein to their common host cell receptor (Du et al., 2009; Hoffmann et al., 2020; Li, 2016; Tortorici et al., 2019; Wrapp et al., 2020). Heavily glycosylated, the spike protein possesses a large ectodomain, a single‐pass transmembrane anchor, and a short intracellular tail (Li, 2016). The ectodomain consists of a receptor‐binding subunit (S1) and a membrane‐fusion subunit (S2; Broer, Boson, Spaan, Cosset, & Corver, 2006; Li, 2016). During virus entry, the S1 subunit recognizes and interacts to host receptors, and further conformational changes in the S2 subunit assist the fusion between the viral envelope protein and the host cell membrane (Du et al., 2009; Li, 2016; Tortorici et al., 2019). S1 subunit has two major domains: the N‐terminal domain (NTD) and the C‐terminal domain (CTD). Depending on the coronavirus type, either CTD or NTD can function as the RBD and bind with diverse proteins and sugars (Li, 2016). Notably, SARS‐CoV and MERS‐CoV utilize CTD to bind their receptors (Kubo, Yamada, & Taguchi, 1994; Li et al., 2005; Ou et al., 2020; N. Wang et al., 2013). Then, the proteases found in the cellular environment cause the cleavage of S proteins, which ensures viral fusion. SARS‐CoV‐2 possesses a furin cleavage site between S1 and S2 subunit that permits efficient cleavage by proteases, including furin that determines viral infectivity and host range (Andersen et al., 2020; Coutard et al., 2020; Ou et al., 2020; Walls et al., 2020). The S protein of the SARS‐CoV‐2 contains a furin‐like cleavage site, which is absent in other coronaviruses of the same clade (Coutard et al., 2020). The S protein also activates the immune response of the host cell toward coronaviruses (Dosch, Mahajan, & Collins, 2009; Walls et al., 2020). Since the S protein of coronaviruses is reported for their vital associations with initial receptor binding and other critical membrane fusion steps, they could be putative targets for blocking the infection. Prior studies revealed that peptide inhibitors to SARS‐CoV‐2 S protein provide promising avenues for SARS‐CoV‐2 treatment by blocking the S protein interaction with ACE2 and thus efficiently preclude the virus entry into human cells. Moreover, these inhibitors can be used as inhaled therapeutics, preventing the virus activation in the lungs (Ou et al., 2020; Walls et al., 2020).
Recently, Wan et al. (2020) and Tai et al. (2020) sequenced and characterized RBD of SARS‐CoV‐2, including its receptor‐binding motif (RBM) that directly associates with ACE2. They also identified some important amino acid residues in SARS‐CoV‐2 RBM (especially Gln493), which facilitates the interactions with human ACE2 and reveals the high capacity of SARS‐Cov‐2 human infection. Moreover, other crucial amino acid residues in SARS‐CoV‐2 RBM, particularly Asn501, contributed SARS‐CoV‐2, gaining some ability for human‐to‐human transmission (Tai et al., 2020; Wan et al., 2020). It was also found that five important amino acids of RBD are conserved in Pangolin‐CoV and SARS‐CoV‐2 (Zhang, Lin, et al., 2020). These findings would give valuable information for finding antigens with improved sensitivity for SARS‐CoV‐2 serological detection and vaccine development.
Envelope protein
E protein of coronavirus is a short polypeptide integral membrane protein that has been involved with different steps of the life cycle of the virus, such as assembly, budding, envelope formation, and pathogenesis (Masters, 2006). It has been reported that the absence or inactivation of E protein could impact either virion morphology or tropism (Khattari et al., 2006; Pervushin et al., 2009). E proteins of coronaviruses have shown cation‐selective ion channel activity and thus have membrane permeabilizing property (Wilson, McKinlay, Gage, & Ewart, 2004). SARS‐CoVs lacking E protein show lower viral titer, immature, and inefficient progenies (Kuo, Hurst, & Masters, 2007), thereby recommending E protein a suitable drug target to prevent viral replication and infection.
3.2. Host cell target
3.2.1. Angiotensin‐converting enzyme 2
ACE2 is a type I membrane protein, which is expressed in the heart, lungs, kidneys, and intestine. An N‐terminal peptidase domain (PD) and a C terminal Collectrin‐like domain (CLD) are usually found in a full‐length ACE2 (Donoghue et al., 2000; H. Zhang et al., 2001). A recent report demonstrated that the ectodomain of the SARS‐CoV‐2S protein interacts with the PD of ACE2 (Wrapp et al., 2020). Like SARS‐CoV, the SARS‐CoV‐2 virus exploits the ACE2 receptor to gain entry into host cells. Human cell‐derived proteases cleave SARS‐CoV‐2 S protein into S1 and S2, where S1 initially interacts its receptor molecule ACE2, and the other fragment, S2, further leads to the membrane fusion after the cleavage by a human cell surface serine protease (TMPRSS2) (Hoffmann et al., 2020). These findings provide valuable insights into the molecular basis for coronavirus recognition and infection. The S protein of SARS‐CoV‐2 is approximately 10‐ to 20‐fold more likely to bind to human ACE2 protein the S protein of SARS‐CoV (Wrapp et al., 2020). This increase in affinity might have facilitated a more favorable person‐to‐person transmission of the SARS‐CoV‐2 infection than the SARS‐CoV (Hoffmann et al., 2020). ACE2 can thus act as a unique adhesion protein molecule for SARS‐CoV‐2 infection and be a promising drug target for the prevention of SARS‐CoV‐2 infection. Disrupting the interaction between spike protein and ACE2 might, therefore, be a possible way of developing a drug against SARS‐CoV‐2. A Phase 1 and Phase 2 clinical trial of human recombinant soluble ACE2 (hrsACE2), a 23‐mer peptide fragment, was carried out recently and suggested to use for the treatment of COVID‐19 (Khan et al., 2017; Zhang, Penninger, et al., 2020). SARS‐CoV‐2 infected Vero‐E6 lines were incubated with hrsACE2 and found that hrsACE2 can preclude the early entrance of SARS‐CoV‐2 infections in host cells perhaps by binding with RBD of S protein (Monteil et al., 2020).
3.2.2. Transmembrane serine protease 2
Although the ACE2 is available in the vascular endothelial cells of all organs, the lungs are more susceptible to SARS‐CoV infection (Hamming et al., 2004). This phenomenon strengthens the possibility of other factors associated with the actual pathogenesis of SARS‐CoV‐2. One such factor which accounts for the viral entry into host cells is TMPRSS2, a well‐known human alveolar and airway protease (Shulla et al., 2011). TMPRSS11a, a TMPRSS2 related protein, has been found to cleave SARS‐CoV S protein and to moderately accelerate viral infections (Kam et al., 2009). TMPRSS2 probably facilitates viral pathogenesis and transmission in two ways: First, TMPRSS2 might activate SARS‐CoV S protein for virus–cell and cell–cell fusion. Second, TMPRSS2 might protect viral recognition by different neutralizing antibodies of the host (Glowacka et al., 2011). Evidence suggests that the entry of SARS‐CoV‐2 into host cells requires both ACE2 and TMPRSS2 (Hoffmann et al., 2020), thereby suggesting that TMPRSS2 might be a potential drug target. Matsuyama et al. (2020) showed that a TMPRSS2‐expressing in VeroE6 cell line is highly susceptible to SARS‐CoV‐2 infection displaying the critical role of TMPRSS2 in the pathogenesis of this viral disease. Previously, Bromhexine hydrochloride, an FDA approved component in mucolytic cough suppressants, showed specific inhibition on TMPRSS2 with no adverse effect in prostate cancer patients and reduction of the progression of metastasis (Lucas et al., 2014). Bromhexine hydrochloride might be repurposed as a TMPRSS2 inhibitor for preventing SARS‐CoV‐2 infection as well (Hoffmann et al., 2020).
3.2.3. Targets on the immune system
Cytokine storm
SARS‐CoV‐2 causes imbalanced and exuberant immune responses termed as cytokine release syndrome or cytokine storm, which triggers lung impairment and reduces the survival of the infected patients (Pedersen & Ho, 2020; Tisoncik et al., 2012). The inflammatory cytokines are usually produced by different macrophages, which are associated with cytokine storm (Luo et al., 2020; Pedersen & Ho, 2020). SARS‐CoV‐2 significantly increases levels of proinflammatory cytokines such as IL‐6, IL‐10, and TNFα in infected patients (Channappanavar et al., 2016; Diao et al., 2020; Nikolich‐Zugich et al., 2020). Furthermore, SARS‐CoV‐2 induced activation of a cluster of differentiation four positive (CD4+) T cells stimulate the generation of T helper cells and secrete interleukin (IL)‐6, and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF). GM‐CSF triggers monocytes to further release of IL‐6 and other factors, resulting in the generation of a cytokine storm. This cytokine storm results in acute respiratory distress syndrome, multiple organ failure, and even death (Chen, Zhang, et al., 2020a; Zhou et al., 2020). Targeted immunomodulation to decrease cytokine storm might be a feasible option to reduce pulmonary inflammation and mortality. Since only antiviral candidates may not be enough to combat such cytokine storm or complications in the respiratory tract, identifying effective targets for immune therapeutics along with antiviral agents could be a potential strategy. Preliminary results from a study suggest that Hydroxychloroquine might have anti‐cytokine storm properties (Yao et al., 2020). Additionally, Lenziluman has been reported to be protective against cytokine storm and neurotoxicity in mouse models. Furthermore, Lenzilumab, an anti‐GM‐CSF monoclonal antibody, can be considered as a viable therapeutic option as COVID‐19 patients (Sterner et al., 2019).
Activation of natural killer cell
The innate immune response itself, without the association of CD8+ T cells and antibodies, is capable of controlling SARS‐CoV (Frieman, Heise, & Baric, 2008). NK cells are the part of innate lymphocyte subsets that mediate anti‐tumor and antiviral responses, and therefore have the potential for clinical use (Abel, Yang, Thakar, & Malarkannan, 2018). Previous studies showed that NK cells showed significant roles in mitigating of SARS‐CoV (Chen et al., 2010; National Research Project for SARS (NSPS), 2004). Recent epidemiological studies showed that SARS‐CoV‐2 infection in children is less frequent and lethal compared to that of adults, which might be due to the increased lymphocyte count, especially NK cells and trained immunity in children (Cristiani et al., 2020). Therefore, the approaches focused on valorizing the innate antiviral immune responses are a possible way to find a remedy against SARS‐CoV‐2. Arabinoxylan treatment resulted in the upregulation of NK cells against neuroblastoma in vitro and in vivo studies (Pérez‐Martínez et al., 2015). Arabinoxylan, therefore, possesses potentiality for the treatment of COVID‐19 patients; however, clinical trials are needed to determine its effectivity.
Activation of autophagy
Autophagy is a natural mechanism of a cell which ensures the maintenance of cellular homeostasis by lysosomal catabolic action, leading the degradation and recycling of intracellular endogenous (macromolecules, abnormal proteins, and damaged organelles) and exogenous (viruses and bacteria) particles (Galluzzi et al., 2017; Levine & Klionsky, 2004). Some viruses have developed strategies to escape autophagic degradation and use host autophagy machinery for their self‐replication (Dong, Dong, & Levine, 2013). The significance of the autophagy process and its therapeutic development for SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 are reviewed by (Yang & Shen, 2020). A recent study showed that MERS‐CoV halts the fusion of autophagosomes and lysosomes, whereas inhibitors like S‐phase kinase‐associated protein 2 induced autophagy, which leads to the reduction of the replication of MERS‐CoV (Gassen et al., 2019). Thus, it seems that a considerable relationship exists between the autophagy machinery and coronavirus infections; therefore, these relationships need to be elucidated in SARS‐CoV‐2 to understand disease prognosis and also to enhance autophagocytosis as a possible treatment corridor.
4. POTENTIAL THERAPEUTICS
Many potential therapeutics are currently in different stages of clinical trials, to find out an effective treatment for COVID‐19, including drugs that have already proven effective for other viral diseases such as human immunodeficiency virus (HIV) and malaria. Drug repurposing could be a rational strategy to develop urgent therapeutics. More than one drug and treatment strategy might prove its worth, and different strategies might work effectively at different infection stages and risk groups. The big challenge will be conducting clinical trials on an urgent basis, determining the effectiveness of different drugs alone or in combination. However, the scientists should take caution to avoid mistakes that occurred during the West African Ebola epidemic, in which haphazard experiments flourished and randomized clinical trials were framed up so late, resulting in little patients in the trial. Besides, developing new drugs or testing procedures may take several years to develop. That is why the World Health Organization (WHO) and other organizations are emphasizing the repurposing therapeutic candidates that have already been approved for other diseases. Researchers are studying antiviral drugs that have performed well in animal studies against SARS‐CoV and MERS‐CoV as well to determine their effectivity against SARS‐CoV‐2. Here, we reviewed some of the potential therapeutics and antivirals, which are under preclinical study and clinical trials. This review would help researchers to get quick and collective information regarding potential drugs.
4.1. Drugs targeting viral entry
As virus entry is a crucial step for the pathogenesis of the COVID‐19, we provide a comprehensive list of repurposed drugs/chemicals that have been reported to block viral entry via different mechanisms (Table 1). Monotherapy or combined therapy of these drugs are in preclinical, Phase 1, Phase 2, or Phase 3 clinical trials. Hoffmann et al. (2020) showed that Camostat mesylate interferes with TMPRSS2‐mediated viral entry, thus reduces the severity of the COVID‐19 disease. Similarly, Arbidol, an antiviral that inhibits the hemagglutinin of the influenza virus and blocks the entry into the cell, is recommended to use against influenza viruses and arboviruses (Kadam & Wilson, 2017; Wang et al., 2004). An open, prospective/retrospective, randomized controlled cohort study is underway at Tongji Hospital, China, to study the safety and efficacy of Arbidol Hydrochloride and Arbidol Hydrochloride combined with interferon atomization in humans (Table 1). In this study, the interventional subjects will be given a standard supportive therapy with Arbidol Hydrochloride (0.2 g, three times a day) or Arbidol Hydrochloride combined with Interferon (PegIFN‐α‐2b) atomization (45 g) (NCT04254874). Trials with Arbidol Hydrochloride, along with other drugs, are also undergoing at Ruijin Hospital, China, and Tongji Hospital, China (NCT04260594, NCT04255017). Moreover, a recent study has demonstrated that a fusion protein made of rhACE2 and an Fc (Fragment crystalization) region of the human immunoglobulin IgG1 shows high‐affinity binding to the receptor‐binding domain of SARS‐CoV‐2 and potently neutralized SARS‐CoV‐2 in vitro, which provides a basis for further drug development (Lei et al., 2020; Monteil et al., 2020). This fusion protein can be considered for further studies in animals and humans to see its effectiveness. Moreover, S protein‐specific monoclonal antibodies can be generated to neutralize the spike protein, which may lead to the inhibition of viral entry. Recently, Tian et al. (2020) identified a monoclonal antibody, CR3022, which binds with the RBD of the spike protein of SARS‐CoV‐2 in vitro. CR3022, therefore, might be a potential drug candidate against SARS‐CoV‐2 infection. Despite the role of ACE2 in viral entry, targeting interruption of ACE2 may not be the best strategy to prevent SARS‐CoV‐2 (Guo et al., 2020) because ACE2 also maintains blood pressure. So, the entry inhibition strategy must be pathogen‐specific rather than host‐specific. Further studies should be carried out to discover potential entry inhibitors that block that viral entry without disturbing the maintenance of the blood pressure. Some other potential therapeutics that block the virus entry into target cells are summarized in Tables 1 and 2.
TABLE 1.
Therapeutics targeting viral entry
| Target | Specific effect/target molecule | Intervention(s) | Status of SARS‐CoV‐2 trial | Sponsor/collaborators | NCT number |
|---|---|---|---|---|---|
| TMPRSS2 | Sialic receptors | DAS181 | Not applicable | Renmin Hospital of Wuhan University, Ansun Biopharma, Inc. | NCT04324489 |
| Serine protease inhibitor | Camostat Mesilate | Phase 1, phase 2 | University of Aarhus | NCT04321096 | |
| Endosome | Endosomal acidification | Chloroquine diphosphate | Phase 2 | Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, Marcus VinÃcius Guimarães de Lacerda, Mayla Gabriela Silva Borba, Wuelton Marcelo Monteiro, Gisely Cardoso de Melo, Fernando Fonseca de Almeida e Val, Felipe Gomes Naveca, Maria Paula Gomes Mourão, Ludmila Abrahão Hajjar, Jorge Souza Mendonça | NCT04323527 |
| Endosomal acidification | Chloroquine or Hydroxychloroquine | Not applicable | University of Oxford | NCT04303507 | |
| Endosomal acidification | Colchicine | Phase 2, Phase 3 | Lucio Manenti, Azienda Ospedaliero‐Universitaria di Parma, Montreal Heart Institute, DACIMA Software | NCT04322565, NCT04322682 | |
| Lysosome |
†1 Bromhexine hydrochloride tablets †2 Arbidol hydrochloride †3 Recombinant human interferon Î ± 2b spray |
Not applicable | Second Affiliated Hospital of Wenzhou Medical University, WanBangDe Pharmaceutical Group Co., Ltd. | NCT04273763 | |
| Lysosome | Hydroxychloroquine | Early Phase 1, Phase 2, Phase 3 | National Institute of Respiratory Diseases, Mexico, Sanofi; Columbia University; National Institute of Respiratory Diseases, Mexico, Sanofi; University of Minnesota; Rambam Health Care Campus, Rabin Medical Center; Shanghai Public Health Clinical Center | NCT04315896, NCT04318444, NCT04318015, NCT04308668, NCT04323631, NCT04261517 | |
| Lysosome | Hydroxychloroquine sulfate | Phase 4 | University Hospital, Akershus | NCT04316377 | |
| Lysosome | Thalidomide | Phase 2 | First Affiliated Hospital of Wenzhou Medical University, Second Affiliated Hospital of Wenzhou Medical University, Wenzhou Central Hospital | NCT04273581 | |
| Spike glycoprotein | Spike glycoprotein |
†1 N‐acetylcysteine + Fuzheng Huayu tablet †2 N‐acetylcysteine + placebo |
Phase 2 | ShuGuang Hospital, Hubei Hospital of Traditional Chinese Medicine, Jingmen No.1 People’s Hospital, Tongji Hospital | NCT04279197 |
| Spike glycoprotein | Nitric oxide | Phase 2 | Massachusetts General Hospital, Xijing Hospital, Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico | NCT04305457, NCT04306393, NCT04312243 | |
| Spike glycoprotein | Nitric oxide 0.5%/nitrogen 99.5% gas for Inhalation | Phase 2 | University of British Columbia, Mallinckrodt | NCT03331445 | |
| Spike glycoprotein |
†1 Abidol hydrochloride †2 Abidol hydrochloride combined with interferon atomization |
Phase 4 | Tongji Hospital | NCT04254874 | |
| Spike glycoprotein | Anti‐ SARS‐CoV‐2 plasma | Phase 2, Phase 3 | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; Mayo Clinic; Foundation IRCCS San Matteo Hospital, OSPEDALE CARLO POMA ASST MANTOVA, OSPEDALE MAGGIORE LODI, OSPEDALE ASST CREMONA; Wuhan Union Hospital, China; Peking Union Medical College Hospital, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology | NCT04323800, NCT04325672, NCT04321421, NCT04264858, NCT04261426 | |
| Spike glycoprotein | Arbidol | Phase 4 | Jieming QU, Ruijin Hospital | NCT04260594 | |
| CD147 | Meplazumab | Phase 1, Phase 2 | Tang‐Du Hospital | NCT04275245 | |
| Spike glycoprotein | Baricitinib | Phase 3 | Hospital of Prato | NCT04320277 | |
| Spike glycoprotein | Human amniotic fluid | Early Phase 1 | University of Utah | NCT04319731 |
Note: †1, †2, and †3 are different arms of parallel intervention.
TABLE 2.
Therapeutics working on multiple targets
| Target | Specific effect/target molecule(s) | Intervention(s) | Status of SARS‐CoV‐2 trial | Sponsor/collaborators | NCT number |
|---|---|---|---|---|---|
| Combined intervention | 3CLpro, interferon receptor (IFNAR) |
†1 Lopinavir/ritonavir †2 Ribavirin †3 Interferon Beta‐1B |
Phase 2 | The University of Hong Kong, Hospital Authority, Hong Kong | NCT04276688 |
| 3CLpro, interferon receptor (IFNAR) |
†1 Xiyanping injection †2 Lopinavir/ritonavir, alpha‐interferon nebulization |
Not applicable | Jiangxi Qingfeng Pharmaceutical Co. Ltd. | NCT04275388 | |
| ACE2 and spike binding complex, Endosomal acidification |
†1 Azithromycin †2 Chloroquine |
Phase 3 | Population Health Research Institute | NCT04324463 | |
| ACE2 and spike binding complex, Endosomal acidification |
†1 Azithromycin †2 Hydroxychloroquine |
Phase 4 | Chronic Obstructive Pulmonary Disease Trial Network, Denmark | NCT04322396 | |
| ACE2 and spike binding complex, Endosomal acidification |
†1 Hydroxychloroquine + azithromycin †2 Hydroxychloroquine |
Phase 3 | Hospital Israelita Albert Einstein, EMS, Hospital do Coracao, Hospital Sirio‐Libanes, Brazilian Research In Intensive Care Network; Hospital do Coracao, Hospital Israelita Albert Einstein, Hospital Sirio‐Libanes, Brazilian Research In Intensive Care Network, EMS | NCT04321278, NCT04322123 | |
| Endosomal acidification, RdRp |
†1 Hydroxychloroquine †2 Remdesivir |
Phase 2, Phase 3 | Oslo University Hospital | NCT04321616 | |
| Spike glycoprotein, 3CLpro |
†1 Carrimycin †2 lopinavir/ritonavir tablets or Arbidol or chloroquine phosphate |
Phase 4 | Beijing YouAn Hospital, Shenyang Tonglian Group Co., Ltd., Institute of Medicine and Biotechnology, Chinese Academy of Medical Sciences, Huangshi Central Hospital, Shenyang Pharmaceutical University, First Affiliated Hospital of Chongqing Medical University, The Second Affiliated Hospital of Harbin Medical University, No. 2 People's Hospital of Fuyang City, First Affiliated Hospital Bengbu Medical College, Renmin Hospital of Wuhan University, The Sixth People's Hospital of Shenyang, Nanyang Central Hospital | NCT04286503 | |
| Spike glycoprotein, 3CLpro |
†1 Abidol hydrochloride †2 Oseltamivir †3 Lopinavir/ritonavir |
Phase 4 | Tongji Hospital | NCT04255017 |
Note: †1, †2, and †3 are different arms of parallel intervention.
4.2. Targeting the virus replication cycle
The replication cycle of coronavirus or the virus‐infected host cells possesses several virus‐specific processes or protein molecules that could be targeted for effective antiviral drug design. An effective antiviral agent might halt the viral progression in the infected cell without being allergic or toxic to surrounding noninfected cells (Zhu, Meng, Wang, & Wang, 2015).
4.2.1. Targeting viral RNA release
We have already discussed above the role of endosomal/lysosomal acidification and the role of their acidic pH‐dependent proteases in the virus life cycle (Yang & Shen, 2020). The inhibition of this pathway might be a potential therapeutic strategy. Several therapeutics such as Chloroquine (CQ), a well‐studied lysosomotropic antimalarial drug, and its derivatives hydroxychloroquine and colchicine would be an option for blocking this target. CQ effectively blocks viral progression by raising endosomal pH needed for membrane fusion between the host cell and viral candidates and releasing RNA from endosomes (Savarino, Boelaert, Cassone, Majori, & Cauda, 2003). Although CQ and hydroxychloroquine were found to be effective against SARS‐CoV‐2 (Gautret et al., 2020; Wang et al., 2020), several recent evidence claimed some unprecedented effects. A recent study reported that hydroxychloroquine (either alone or in combination with azithromycin) showed no positive outcome in patients with COVID‐19 (Magagnoli et al., 2020), instead was linked with increased overall mortality (27.8% in hydroxychloroquine and 22.1% in hydroxychloroquine plus azithromycin versus 11.4% in the control group). A multinational, observational, real‐world study of patients with COVID‐19 also claimed that hydroxychloroquine or chloroquine showed no evidence of benefit, rather it was associated with an increase in the risk of ventricular arrhythmias and a greater hazard for in‐hospital death with COVID‐19 (Mehra, Desai, Ruschitzka, & Patel, 2020). Moreover, a recent systematic review found no difference between the hydroxychloroquine treated group and an untreated group of COVID‐19 patients (Sarma et al., 2020). WHO had recently postponed further clinical trials on hydroxychloroquine due to unexpected results in previous trials, however, this repurposed drug has been allowed for re‐evaluation. All of this evidence points to the significance of waiting for the final results of ongoing prospective, randomized, controlled studies before considering these drugs as a therapeutic option for COVID‐19.
4.2.2. Protease inhibitors
Proteases have a crucial role in many signaling pathways and represent promising drug targets fora wide range of diseases, including viral diseases. A few protease blockers are currently under Phase 1, Phase 2, or Phase 3 clinical trials as potential drugs for treating COVID‐19 (Tables 1 and 2). ASC09/Ritonavir, Lopinavir/Ritonavir, ASC09F + Oseltamivir, and Ritonavir + Oseltamivir are some protease inhibitor drug candidates that inhibit 3CLpro and prevent subsequent progression of replication (Table 1). For example, monotherapy or combination of Lopinavir and Ritonavir has been studied against MERS‐CoV both in vitro and in an animal model and found to be effective (Chan et al., 2015; de Wilde et al., 2014). Studies showed that the combination of Lopinavir and Ritonavir with Ribavirin and Interferon alfa (IFN‐α) resulted in the survival of MERS‐patients through viral clearance (Kim, Won, Kee, Jung, & Jang, 2016). Some investigators are operating randomized, open‐label trials to evaluate and compare the efficacy and safety of Lopinavir and Ritonavir for COVID‐19 patients (Table 3). A recent case report claimed that a 54‐year‐old patient administered with lopinavir/ritonavir showed a significant reduction of viral loads with no or little coronavirus titers (Lim et al., 2020). A recent clinical trial (ChiCTR2000029308), however, showed that lopinavir–ritonavir treatment caused no benefit to hospitalized patients with severe COVID‐19 compared to standard care (Cao et al., 2020). Treatment with lopinavir–ritonavir was not associated with time to clinical improvement, mortality at 28 days, detectable viral RNA, and gastrointestinal adverse events compared with the standard‐care group (Cao et al., 2020). These contradictory yet promising findings highlight the importance of conducting clinical trials before using a drug as a therapeutic candidate.
TABLE 3.
Therapeutics intervening viral replication process
| Target | Specific effect/target molecule | Intervention(s) | Status of SARS‐CoV‐2 trial | Sponsor/collaborators | NCT number |
|---|---|---|---|---|---|
| Protease | 3CLpro |
†1 ASC09/ritonavir †2 lopinavir/ritonavir |
Not applicable | First Affiliated Hospital of Zhejiang University, Ascletis Pharmaceuticals Co., Ltd. | NCT04261907 |
| 3CLpro |
†1 ASC09F + Oseltamivir †2 Ritonavir + Oseltamivir †3 Oseltamivir |
Phase 3 | Tongji Hospital | NCT04261270 | |
| 3CLpro | Darunavir and Cobicistat | Phase 3 | Shanghai Public Health Clinical Center | NCT04252274 | |
| 3CLpro | Ganovo + ritonavir ± interferon nebulization | Phase 4 | The Ninth Hospital of Nanchang, Ascletis Pharmaceuticals Co., Ltd. | NCT04291729 | |
| 3CLpro | Lopinavir/ritonavir | Phase 3 | Darrell Tan, St. Michael's Hospital, Toronto; Jiangxi Qingfeng Pharmaceutical Co. Ltd. | NCT04321174, NCT04295551 | |
| 3CLpro |
†1 Lopinavir/ritonavir †2 Hydroxychloroquine sulfate |
Phase 2 | Asan Medical Center | NCT04307693 | |
| 3CLpro |
†1 Lopinavir/ritonavir †2 Hydroxychloroquine sulfate †3 Baricitinib (Janus kinase inhibitor) †4 Sarilumab (anti‐IL‐6 receptor) |
Phase 2 | Lisa Barrett, Nova Scotia Health Authority, Dalhousie University | NCT04321993 | |
| RdRp | RdRp |
†1 Favipiravir combined with Tocilizumab †2 Favipiravir †3 Tocilizumab |
Not applicable | Peking University First Hospital | NCT04310228 |
| RdRp | Remdesivir | Phase 3 | Gilead Sciences; U.S. Army Medical Research and Development Command; Institut National de la Santé Et de la Recherche Médicale, France; Capital Medical University, Chinese Academy of Medical Sciences; Capital Medical University; Gilead Sciences; National Institute of Allergy and Infectious Diseases (NIAID) | NCT04323761, NCT04302766, NCT04315948, NCT04252664, NCT04257656, NCT04292899, NCT04292730, NCT04280705 | |
| RdRp | Ribavirin | Not applicable | Jiangsu Famous Medical Technology Co., Ltd. | NCT04306497 | |
| RdRp | Galidesivir (BCX4430) | Phase 1 | BioCryst Pharmaceuticals, National Institute of Allergy and Infectious Diseases (NIAID) | NCT03891420 | |
| RdRp | Triazavirin | Phase 3 | Health Commission of Heilongjiang province | ChiCTR2000030001 |
Note: †1, †2, †3, and †4 are different arms of parallel intervention.
4.2.3. Replication blocker
RdRp is a vital enzyme of the viral replication machinery; hence, RdRp blockers could be effective antiviral therapy against SARS‐CoV‐2 infection. Depending on their mechanism of action, RdRp inhibitors are of two types, that is, (a) nucleoside and nucleotide structural analogs which mimic the structure of the natural nucleoside triphosphate substrates and could interact competitively in the active site of RdRp polymerase and (b) non‐nucleoside inhibitors are allosteric inhibitors which interact in a noncompetitive fashion for the substrates (Brown, 2009; Velkov et al., 2014). Favipiravir (T‐705) is a broad‐spectrum prodrug that is metabolized into an active ribofuranosyl triphosphate derivative that selectively inhibits the viral RdRp polymerase activity (Velkov et al., 2014). Favipiravir was shown to inhibit the replication of the respiratory syncytial virus and also been proven effective against influenza in Japan (Delang, Abdelnabi, & Neyts, 2018; Furuta, Komeno, & Nakamura, 2017), which indicates that it might be effective against SARS‐CoV‐2. A multicenter, randomized, and controlled clinical trial study with 150 participants is underway with Favipiravir combined with Tocilizumab for the treatment of COVID‐19 at Peking University First Hospital (Table 3). Recently, a prospective, randomized, controlled, open‐label multicenter trial (ChiCTR2000030254) involving adult COVID‐19 patients treated with Favipiravir versus Arbidol showed that Favipiravir increased clinical recovery rate, improved the latency to relief for pyrexia and cough along with mild and manageable adverse effects (Chen, Xiong, Bao, & Shi, 2020). Furthermore, Cai et al. (2020) studied the effects of Favipiravir versus Lopinavir/ritonavir for the treatment of COVID‐19. There were two arms (Favipiravir + Interferon‐α versus Lopinavir/ritonavir) in this open‐label control study. The 45 patients registered in the control arm (Lopinavir/ritonavir) and the 35 patients in the Favipiravir arm (Favipiravir + Interferon‐α) where all baseline characteristics were compared between the two arms. The Favipiravir arm showed significant improvement in chest imaging, associated with faster viral clearance with lower adverse events compared with the control arm (Cai et al., 2020). Remdesivir, an adenosine nucleoside analog prodrug and developed by Gilead Sciences (Foster City, CA) against the Ebola virus, incorporates into nascent viral RNA chains resulting in the premature termination of RNA synthesis (Wang, Cao, et al., 2020). Monotherapy and combination therapy of Remdesivir with CQ or Interferon‐beta (IFN‐β) halted SARS‐CoV‐2 replication and treated patients were clinically recuperated (Sheahan et al., 2020; Wang, Zhang, et al., 2020). A Phase 3 randomized, double‐blind, placebo‐controlled, multicenter study of Remdesivir in hospitalized adult patients with severe COVID‐19 is completed (NCT04257656). The primary endpoint of the trial concluded that patients with COVID‐19 receiving Remdesivir showed a faster time to clinical improvement (numerically, not statistically significant) compared to the patients receiving the placebo group (Wang, Cao, et al., 2020). Moreover, a double‐blind, randomized, placebo‐controlled clinical trial (NCT04280705) evidenced that intravenous Remdesivir was efficacious compared to placebo in reducing the time to recovery with lower respiratory tract infection (Beigel et al., 2020). Recently, the FDA approved the emergency use of Remdesivir as an experimental drug. On the other hand, Ribavirin, a synthetic purine nucleoside derivative could interfere in the guanosine triphosphate formation, hinders capping of viral mRNA, and thus inhibits viral RNA‐dependent RNA polymerase activity (Khalili, Zhu, Mak, Yan, & Zhu, 2020). It could be used as a broad‐spectrum antiviral therapeutic agent that has already been reported against a wide range of DNA and RNA viruses such as paramyxovirus, Influenza A and B, Hepatitis C, respiratory syncytial virus, parainfluenza, and HIV (Elfiky, 2020; Khalili et al., 2020; Tam, Lau, & Hong, 2001; Tan et al., 2004). Several phases of clinical trials are undergoing to study the efficacy of Remdesivir and Ribavirin for the treatment of SARS‐CoV‐2 as alone or in combination with other drugs (Table 3; Khalili et al., 2020).
4.3. Drugs targeting the inflammatory responses and cytokine storm
Inflammation is an obvious outcome of SARS‐CoV‐2; however, no effective therapeutics targeting the inflammatory responses for SARS‐CoV‐2 has been approved to date. Fu, Cheng, and Wu (2020) reviewed the potential mechanism of Fc receptor (FcR) blockers such as intravenous immunoglobulin, anti‐Fc specific antibodies, small molecules, and so on, which might be a therapeutic tool for reducing SARS‐CoV‐2 induced pulmonary inflammation. Moreover, anti‐inflammatory drugs or corticosteroids might also effectively alleviate early proinflammatory response to reduce SARS‐CoV‐2‐induced inflammatory responses by blocking FcR activation. Besides, the administration of intravenous immunoglobulin against FcR could be effective for an urgent remedy against pulmonary inflammation associated with severe lung injury as it can effectively block FcR activation. These approaches may also be applied with corticosteroids or systemic anti‐inflammatory drugs (Shang, Zhao, Hu, Du, & Cao, 2020), which needs further clinical trials to study the effectiveness against SARS‐CoV‐2 infection (Russell, Millar, & Baillie, 2020).
Another potential option for reducing inflammation is Siltuximab, a chimeric monoclonal antibody (mAb) that binds to and halts the effect of IL‐6 (Sarosiek, Shah, & Munshi, 2016; Chen et al., 2020). Recently, a study in COVID‐19 patients showed that intravenous administration of Siltuximab improved clinical conditions of 33% patients, stabilized or demonstrated no clinically relevant changes in 43% of patients, whereas condition deteriorated in 24% patients (Gritti et al., 2020). These results exhibit the potential role of Siltuximab in treating patients with SARS‐CoV‐2 infection (Gritti et al., 2020). Furthermore, an observational case–control study with 50 COVID‐19 patients with serious respiratory complications is ongoing in Italy to determine the effectivity of Siltuximab (NCT04322188). Another group of researchers (di Giambenedetto et al., 2020) used Tocilizumab, a humanized anti‐human interleukin‐6 receptor antibody of the IgG1 subclass, for thwarting or treating the cytokine storm in three patients of COVID‐19 and found promising results such as reduction of fever, C‐reactive proteins and quick relief of respiratory symptoms. A clinical trial is undergoing to test whether an anti‐IL6 treatment can be effective in reducing the SARS‐CoV‐2‐induced cytokine storm and improving lung function (NCT04315480). Some other therapeutics which have anti‐inflammatory and immunomodulation property are summarized in Table 4.
TABLE 4.
Therapeutic agents with anti‐inflammatory and immunomodulatory actions
| Target | Specific effect/target molecule | Intervention(s) | Status of SARS‐CoV‐2 trial | Sponsor/collaborators | NCT number |
|---|---|---|---|---|---|
| Immune system | Immunomodulatory | Mesenchymal stem cell (MSC) | Phase 1, Phase 2 | Beijing 302 Hospital, Innovative Precision Medicine Group (IPM), Hangzhou, China., Wuhan Huoshenshan Hospital, Wuhan, China, Tianjin Haihe Hospital, VCANBIO CELL & GENE ENGINEERING CORP., LTD, China, Shenzhen Third People's Hospital, Fifth Affiliated Hospital, Sun Yat‐Sen University | |
| Immunomodulatory | NestCell® | Phase 1 | Azidus Brasil, Cellavita Pesquisa CientÃfica Ltda, Hospital Vera Cruz | NCT04315987 | |
| Immunomodulatory | UC‐MSCs | Phase 2 | ZhiYong Peng, Tuohua Biological Technology Co. Ltd, Zhongnan Hospital; Wuhan Union Hospital, China, Wuhan Hamilton Biotechnology Co., Ltd, China. | NCT04269525, NCT04273646 | |
| Immunomodulatory | WJ‐MSCs | Phase 1 | Stem Cells Arabia | NCT04313322 | |
| Immune system | Angiotensin II receptor | Losartan | Phase 2 | University of Minnesota | NCT04312009, NCT04311177 |
| Cytokine | Dexamethasone | Phase 4 | Dr. Negrin University Hospital, Li Ka Shing Knowledge Institute | NCT04325061 | |
| Cytokine | Methylprednisolone | Phase 2, Phase 3, Phase 4 | Beijing Chao Yang Hospital; Tongji Hospital; Peking Union Medical College Hospital, Zhongda Hospital, Zhongnan Hospital, Renmin Hospital of Wuhan University; University of Trieste | NCT04273321, NCT04263402, NCT04244591, NCT04323592 | |
| Cytokine | MSCs‐derived exosomes | Phase 1 | Ruijin Hospital, Shanghai Public Health Clinical Center, Wuhan Jinyintan Hospital, Wuhan, China, Cellular Biomedicine Group Ltd. | NCT04276987 | |
| Eicosanoids and nuclear factor‐kappa B (NF‐kB) | Escin | Phase 2, Phase 3 | University of Catanzaro, Azienda Ospedaliera Pugliese Ciaccio, Azienda Ospedaliera Policlinico "Mater Domini" | NCT04322344 | |
| Infected human cells | NK cells, IL15‐NK cells, NKG2D CAR‐NK cells, ACE2 CAR‐NK cells,NKG2D‐ACE2 CAR‐NK cells | Phase 1, Phase 2 | Chongqing Public Health Medical Center, Chongqing Sidemu Biotechnology Technology Co., Ltd., Xinxiang medical university, First Affiliated Hospital of Xinjiang Medical University | NCT04324996, NCT04280224 | |
| Injured cell components | CD24Fc | Phase 3 | OncoImmune, Inc. | NCT04317040 | |
| Interferon gamma (IFNy) | Emapalumab | Phase 2, Phase 3 | Swedish Orphan Biovitrum | NCT04324021 | |
| Interferon receptor (IFNAR) |
†1 Recombinant human interferon Alpha‐1b †2 thymosin alpha 1 |
Phase 3 | Shanghai Jiao Tong University School of Medicine | NCT04320238 | |
| Interferon receptor (IFNAR) | Recombinant human interferon Î ± 1Î2 | Early Phase 1 | Tongji Hospital | NCT04293887 | |
| Interleukin‐1 type 1 receptor | Anakinra | Phase 2, Phase 3 | Swedish Orphan Biovitrum | NCT04324021 | |
| Interleukin‐1 type 1 receptor |
†1 RoActemra iv †2 RoActemra sc †3 Kevzara sc |
Phase 2 | Marius Henriksen, Lars Erik Kristensen, Frederiksberg University Hospital | NCT04322773 | |
| Interleukin‐1 type 1 receptor | Sarilumab | Phase 2, Phase 3 | Assistance Publique ‐ HÃ'pitaux de Paris; Regeneron Pharmaceuticals, Sanofi | NCT04324073, NCT04315298 | |
| Interleukin‐6 receptor | Tocilizumab | Phase 2, Phase 3 | Università Politecnica delle Marche, Azienda Ospedaliera Ospedali Riuniti Marche Nord; Hoffmann‐La Roche, National Cancer Institute, Naples; Tongji Hospital, Hubei Xinhua Hospital, Wuhan No.1 Hospital, Wuhan central hospital | NCT04315480, NCT04320615, NCT04317092, NCT04306705 | |
| Interleukin‐6, TNFα | Aviptadil | Phase 2 | NeuroRx, Inc., Relief Therapeutics Holding SA | NCT04311697 | |
| Programmed cell death protein 1 |
†1 Programmed death‐1 (PD‐1) blocking antibody +standard treatment †2 Thymosin + standard treatment |
Phase 2 | Southeast University, China | NCT04268537 | |
| Prostaglandins | Naproxen | Phase 3 | Assistance Publique ‐ HÃ'pitaux de Paris | NCT04325633 | |
| Sphingosine 1‐phosphate (S1P) receptor | Fingolimod | Phase 2 | First Affiliated Hospital of Fujian Medical University | NCT04280588 | |
| Toll‐like receptor (TLR) | PUL‐042 Inhalation Solution | Phase 2 | Pulmotect, Inc. | NCT04313023, NCT04312997 |
Note: †1, †2, and †3 are different arms of parallel intervention.
5. POTENTIAL VACCINES AND THEIR DEVELOPMENT
A vaccine is an ultimate hope to prevent and reduce the spread of COVID‐19. A vaccine can be either prophylactic, which prevents future infection or therapeutic, which is used to treat diseases such as cancer or infectious diseases (Frazer, 2014; Guo et al., 2013). The whole pathogen (either killed or live‐attenuated), or parts of the pathogen, such as nucleic acid, proteins, or peptides, can be used as vaccines (Haque, Shah, Paul, & Barua, 2020; Shah & Md Kawsar, 2015). Whole virus vaccines have been widely studied and showed protection against the influenza virus (Fiore, Bridges, & Cox, 2009), the human papillomavirus, and the chickenpox virus (Liesegang, 2009); whereas, subunit and peptide‐based vaccines have shown immunity in vitro (Khan, Hossain, Rakib‐Uz‐Zaman, & Morshed, 2014) and animal studies (Islam, Clemens, & Qadri, 2018). There were efforts to develop vaccines against SARS‐CoV and MERS‐CoV, which were tested in animal models (Gao et al., 2003; Kim et al., 2014). However, there is no benign and operational vaccine available against SARS‐CoV until now. Development of vaccines against SARS‐CoV after the SARS outbreak in 2002–2004 was attempted (Greenough et al., 2005; Roberts et al., 2006; Tripp et al., 2005); however, the efforts did not result in finding any effective vaccines until now. During MERS prevalence, it was believed that SARS‐CoV would provide a foundation and a template for developing vaccines against MERS‐CoV infection (Jiang, Lu, & Du, 2013). However, only one MERS‐CoV DNA vaccine has so far completed a Phase 1 clinical trial (Yong, Ong, Yeap, Ho, & Tan, 2019). Several more viral vectored‐MERS‐CoV vaccines are in progress (Yong et al., 2019) which were reviewed by Zumla, Chan, Azhar, Hui, and Yuen (2016).
A suitable vaccine of SARS‐CoV‐2 can prevent the spread of the current pandemic COVID‐19 and can save millions of lives and billions of money. Currently, different research groups around the world are working with more than 100 candidate vaccines against SARS‐CoV‐2 based on (1) inactivated or weakened whole virus, (2) nucleic acid, (3) viral vector, and (4) protein subunit or virus‐like particles (Figure 3), which are in the preclinical evaluation for SARS‐CoV‐2 (Amanat & Krammer, 2020; Chen, Xiong, Bao, & Shi, 2020). As of May 2020, some vaccines are under different phases of clinical trials (Table 5). Five of those vaccines are in Phase 1 clinical trials, six are in Phase 2 trials and one vaccine candidate is in Phase 3 clinical trials (Table 5). Those candidate vaccines are DNA based (2), inactivated virus (2), modified antigen‐presenting cell (APC) (3), nonreplicating virus (2), and protein subunit based (1) (Table 5). The nonreplicating virus‐based vaccine, namely ChAdOx1 nCoV‐19, developed by the Consortium of the Jenner Institute, Oxford Biomedica, University of Oxford, which is under phase three clinical trial is considered as the most promising vaccine candidate so far. ChAdOx1 construct contains S protein that can elicit an immune response in the body, which might prevent subsequent infection of SARS‐CoV‐2 (Lane, 2020). The Phase 1 trial initiated in April where 1,000 plus immunizations have been accomplished and follow‐up is currently continuing. Now commencing Phase 2 and Phase 3 trials to assess how well the vaccine provokes immune responses in older adults, and to examine whether it can render protection in the wider population (http://www.ox.ac.uk/news/2020-05-22-oxford-covid-19-vaccine-begin-phase-iiiii-human-trials). Another vaccine is the mRNA‐1273 vaccine, a synthetic strand of mRNA, which translates the prefusion‐stabilized viral spike protein. The mRNAs, after intramuscular injection, are predicted to generate antiviral responses toward the spike protein of SARS‐CoV‐2. Furthermore, the synthesis of the lipid nanoparticle‐encapsulated mRNA vaccine does not require the virus‐like conventional vaccines; therefore, these are harmless and ready to be tested (NCT04283461). However, no vaccine has completed any clinical trial yet, and no vaccines are available to prevent SARS‐CoV‐2 infection (Amanat & Krammer, 2020; Chen, Zhang, et al., 2020b).
FIGURE 3.
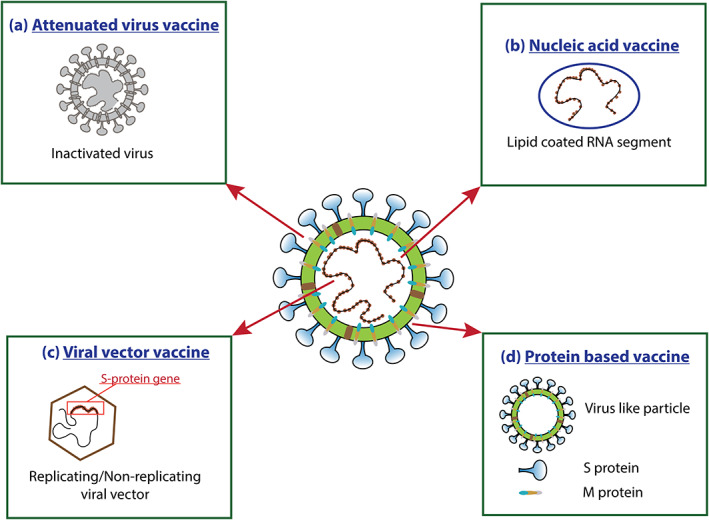
Possible strategies for vaccine design and development. (a) Attenuated Virus Vaccine: Attenuation of the virus by chemicals, such as formaldehyde, or heat; (b) Nucleic acid vaccine: Contains the genetic material only; (c) Viral vector vaccine: A genetically modified virus that can generate SARS‐CoV‐2 proteins in the body; (d) Protein‐based vaccine: protein shells or fragments of proteins that imitate the virus's outer coat
TABLE 5.
Potential vaccine candidates for SARS‐CoV‐2 that are under different stages of clinical trials
| Technology | Vaccine | Status of trial | Sponsor/collaborators | NCT number |
|---|---|---|---|---|
| D NA‐based vaccine | INO‐4800 | Phase 1 | Inovio Pharmaceuticals | NCT04336410 |
| bacTRL‐Spike | Phase 2 | Symvivo Corporation, University of British Columbia, Dalhousie University | NCT04334980 | |
| Inactivated virus | CoronaVac (SARS‐CoV‐2 inactivated vaccine) | Phase 1/2 | Sinovac Biotech Co. | NCT04352608, NCT04383574 |
| V‐SARS | Phase 1/2 | Immunitor LLC | NCT04380532 | |
| Modified APC | LV‐SMENP‐DC | Phase 1/2 | Shenzhen Geno‐Immune Medical Institute | NCT04276896 |
| AV‐COVID‐19 | Phase 1/2 | Aivita Biomedical, Inc. | NCT04386252 | |
| Covid‐19/aAPC vaccine | Phase 1 | Shenzhen Geno‐Immune Medical Institute | NCT04299724 | |
| Nonreplicating viral vector | AZD1222 (ChAdOx1 nCoV‐19) | Phase 1/2/3 | Consortium of the Jenner Institute, Oxford Biomedica, University of Oxford | NCT04324606, EudraCT 2020–001072‐15, EudraCT 2020–001228‐32 |
| Ad5‐nCoV | Phase 1/2 | CanSino Biologics Inc., Institute of Biotechnology and so on | NCT04313127, NCT04341389, NCT04398147, ChiCTR2000031781, ChiCTR2000030906 | |
| Protein subunit | NVX‐CoV2373 (SARS‐CoV‐2 rS) | Phase 1 | Novavax | NCT04368988 |
| SCB‐2019 | Phase 1 | Clover Biopharmaceuticals | NCT04405908 | |
| RNA‐based vaccine | BNT162 | Phase 1/2 | Biontech RNA Pharmaceuticals GmbH, Pfizer | NCT04380701, NCT04368728, EudraCT 2020‐001038‐36 |
| mRNA‐1273 | Phase 1 | Moderna, NIAID | NCT04283461 | |
| Repurposed vaccine | Bacille Calmette‐Guerin (BCG) vaccine | Phase 3/4 | University Medical Center Utrecht, Radboud University and so on | NCT04328441, NCT04350931, NCT04362124, NCT04379336, NCT04347876, NCT04327206, NCT04369794, NCT04373291, NCT04384549, NCT04348370, NCT04384614, NCT04387409 |
| Measles‐mumps‐rubella (MMR) vaccine | Phase 3 | Kasr El Aini Hospital | NCT04357028 |
6. PASSIVE AND MONOCLONAL ANTIBODY THERAPY
In the passive antibody therapy approach, the antibodies from the blood plasma or serum (convalescent plasma or sera) of people who have recuperated from SARS‐CoV‐2 infection are used to enhance the immunity of newly infected patients and those at risk of the disease, such as frontline caregivers or essential service providers. These serum antibodies can bind to and neutralize SARS‐CoV‐2 (Casadevall & Pirofski, 2020). The precise timing of collecting blood from the recovered patient is crucial to boost the immunity of the patients (Casadevall & Pirofski, 2020). Such approaches of using convalescent sera or plasma were used effectively in the past during mumps and measles epidemics (Casadevall & Pirofski, 2020). This approach has already been applied in China, where five SARS‐CoV‐2 infected, severely ill patients with acute respiratory distress syndrome were treated with convalescent plasma transfusion from five different recovered patients (Chen, Zhang, et al., 2020a; Teixeira da Silva, 2020). This initiative showed promising results and improved the clinical condition of the treated patients (Shen et al., 2020). However, the limited number of participants in this study precludes a definitive statement about the potential effectiveness of administering convalescent plasma. It emphasizes the need for an evaluation in clinical trials with a larger sample size and proper randomized study design (Shen et al., 2020). Recently, convalescent plasma therapy has started to treat 245 COVID‐19 patients (Casadevall & Pirofski, 2020); this study will focus on the feasibility and effectiveness of plasma therapy.
Another possible treatment option is monoclonal antibody therapy. Bevacizumab, a humanized monoclonal antibody, blocks vascular endothelial growth factor (VEGF) to halt the angiogenesis in cancer patients (Ellis, 2006). A Phase 3 randomized, double‐blind, placebo‐controlled, multicenter study of Remdesivir in hospitalized adult patients with severe COVID‐19 has been completed (NCT04257656). A multicenter randomized controlled clinical trial is undergoing at Qilu Hospital of Shandong University to check the safety and efficacy of bevacizumab in severe or critical patients with COVID‐19 (NCT04305106). COVID‐19 patients encounter severe hypoxia, which can upregulate VEGF. Bevacizumab might reduce the VEGF level and reduce the severity of the disease (Wang, Cai, et al., 2004).
7. CONCLUSION AND FUTURE PERSPECTIVES
As COVID‐19 spreads rapidly, the shortest and fastest approaches such as drug repurposing could be of the right choice that needs to be employed to discover potential therapeutics. Currently, some repurposed drugs have shown therapeutic promise, and thus Favipiravir, for instance, has already received temporary approval for use in COVID‐19 patients. Other compounds with antiviral potential, for example, β‐d‐N4‐hydroxycytidine showing inhibitory effects against Chikungunya Virus, also need to be evaluated against SARS‐CoV‐2. However, recent hype on hydroxychloroquine or chloroquine, for example, as COVID‐19 therapy should be handled carefully, as some studies claimed unprecedented side‐effects posed by these anti‐malarial drugs (Mehra et al., 2020). Simultaneously, the path to vaccine development also needs to be minimized for long‐term protection, of course addressing all the possible side‐effects. As viruses are prone to frequent mutation, vaccines based on a single target might not work for a longer time. Recently, in silico studies identified several protein subunits as vaccine candidates, combining them into a single vaccine or multiple epitope vaccines might give better results against SARS‐CoV‐2.
In this review, we revisited the current knowledge on the potential drug targets of therapeutic promise against SARS‐CoV‐2 infection and highlighted the application of the drug repurposing approach. The possible therapeutic options that are currently under clinical and preclinical studies have been discussed. We also compiled the current clinical trials mostly based on repurposing the therapeutic agents formerly designed for other indications that have been rapidly started during the early phase of the pandemic and are currently undergoing. Although far from practical application, significant progress has been made in vaccine development. Overall, this current effort leaves some valuable information on prospective druggable targets and current options of possible therapeutic as well as preventive strategies, providing a comprehensive and systematic guideline for those who are dedicated to COVID‐19 research.
Beyond the approaches that have been addressed in this review, the strategies aimed at priming the host immune responses are of great potential as individuals with a compromised immune system are particularly vulnerable to COVID‐19. Moreover, along with viral‐specific therapeutics, immunomodulatory and anti‐inflammatory agents acting against nonspecific inflammatory responses also need to be focused. The lessons learned from this emerging pandemic can help us prepare in advance for any possible future epidemics. As we live in the era of big data, systematic and multidisciplinary approaches need to be employed to find drug targets and repurposing drugs for instant drug discovery.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This review did not receive any financial support from a specific project. M. A. H. acknowledges postdoctoral support (Korea Research Fellowship Program, #2018H1D3A1A01074712) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
Sohag AAM, Hannan MA, Rahman S, et al. Revisiting potential druggable targets against SARS‐CoV‐2 and repurposing therapeutics under preclinical study and clinical trials: A comprehensive review. Drug Dev Res. 2020;81:919–941. 10.1002/ddr.21709
Funding information Ministry of Science, ICT and Future Planning; National Research Foundation of Korea; Korea Research Fellowship Program, Grant/Award Number: 2018H1D3A1A01074712
Contributor Information
Abdullah Al Mamun Sohag, Email: sohag2010bmb.sust@gmail.com.
Md Abdul Hannan, Email: hannanbmb@bau.edu.bd.
REFERENCES
- Abel, A. M. , Yang, C. , Thakar, M. S. , & Malarkannan, S. (2018). Natural killer cells: Development, maturation, and clinical utilization. Frontiers in Immunology, 9, 1869. 10.3389/fimmu.2018.01869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedeji, A. O. , Singh, K. , Kassim, A. , Coleman, C. M. , Elliott, R. , Weiss, S. R. , & Sarafianos, S. G. (2014). Evaluation of SSYA10‐001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrobial Agents and Chemotherapy, 58(8), 4894–4898. 10.1128/aac.02994-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat, F. , & Krammer, F. (2020). SARS‐CoV‐2 vaccines: Status report. Immunity, 52(4), 583–589. 10.1016/j.immuni.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, K. G. , Rambaut, A. , Lipkin, W. I. , Holmes, E. C. , & Garry, R. F. (2020). The proximal origin of SARS‐CoV‐2. Nature Medicine, 26(4), 450–452. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, S. J. , Johnson, C. K. , Greig, D. J. , Kramer, S. , Che, X. , Wells, H. , & Goldstein, T. (2017). Global patterns in coronavirus diversity. Virus Evolution, 3(1), vex012. 10.1093/ve/vex012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour, H. M. , Elkhatib, W. F. , Rahman, M. M. , & Elshabrawy, H. A. (2020). Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens, 9(3), E186. 10.3390/pathogens9030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez‐Santos, Y. M. , St John, S. E. , & Mesecar, A. D. (2015). The SARS‐coronavirus papain‐like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Research, 115, 21–38. 10.1016/j.antiviral.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto, N. , Jukneliene, D. , Ratia, K. , Chen, Z. , Mesecar, A. D. , & Baker, S. C. (2005). The papain‐like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. Journal of Virology, 79(24), 15189–15198. 10.1128/jvi.79.24.15189-15198.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel, J. H. , Tomashek, K. M. , Dodd, L. E. , Mehta, A. K. , Zingman, B. S. , Kalil, A. C. , & Lane, H. C. (2020). Remdesivir for the treatment of Covid‐19—Preliminary report. The New England Journal of Medicine. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- Belouzard, S. , Chu, V. C. , & Whittaker, G. R. (2009). Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proceedings of the National Academy of Sciences of the United States of America, 106(14), 5871–5876. 10.1073/pnas.0809524106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer, R. , Boson, B. , Spaan, W. , Cosset, F. L. , & Corver, J. (2006). Important role for the transmembrane domain of severe acute respiratory syndrome coronavirus spike protein during entry. Journal of Virology, 80(3), 1302–1310. 10.1128/jvi.80.3.1302-1310.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N. A. (2009). Progress towards improving antiviral therapy for hepatitis C with hepatitis C virus polymerase inhibitors. Part I: Nucleoside analogues. Expert Opinion on Investigational Drugs, 18(6), 709–725. 10.1517/13543780902854194 [DOI] [PubMed] [Google Scholar]
- Bzówka, M. , Mitusińska, K. , Raczyńska, A. , Samol, A. , Tuszyński, J. , & Góra, A. (2020). Molecular dynamics simulations indicate the COVID‐19 Mpro is not a viable target for small‐molecule inhibitors design. bioRxiv 2020.2002.2027.968008. 10.1101/2020.02.27.968008%J [DOI] [PMC free article] [PubMed]
- Cai, Q. , Yang, M. , Liu, D. , Chen, J. , Shu, D. , Xia, J. , & Liu, L. (2020). Experimental treatment with Favipiravir for COVID‐19: An open‐label control study. Engineering. 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , & Wang, C. (2020). A trial of Lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. The New England Journal of Medicine, 382, 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall, A. , & Pirofski, L. A. (2020). The convalescent sera option for containing COVID‐19. The Journal of Clinical Investigation, 130(4), 1545–1548. 10.1172/jci138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. F. , Yao, Y. , Yeung, M. L. , Deng, W. , Bao, L. , Jia, L. , & Yuen, K. Y. (2015). Treatment with Lopinavir/ritonavir or interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset. The Journal of Infectious Diseases, 212(12), 1904–1913. 10.1093/infdis/jiv392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , Fehr, A. R. , Vijay, R. , Mack, M. , Zhao, J. , Meyerholz, D. K. , & Perlman, S. (2016). Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host & Microbe, 19(2), 181–193. 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Zhang, X. R. , Ju, Z. Y. , & He, W. F. (2020). Advances in the research of cytokine storm mechanism induced by Corona virus disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi, 36(0), E005. 10.3760/cma.j.cn501120-20200224-00088 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Zhang, Y. , Huang, J. , Yin, P. , Cheng, Z. , Wu, J. , & Wang, X. (2020). Favipiravir versus Arbidol for COVID‐19: A randomized clinical trial. medRxiv 2020.2003.2017.20037432. doi: 10.1101/2020.03.17.20037432%J. [DOI]
- Chen, J. , Lau, Y. F. , Lamirande, E. W. , Paddock, C. D. , Bartlett, J. H. , Zaki, S. R. , & Subbarao, K. (2010). Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. Journal of Virology, 84(3), 1289–1301. 10.1128/jvi.01281-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Xiong, J. , Bao, L. , & Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID‐19. The Lancet Infectious Diseases, 20(4), 398–400. 10.1016/s1473-3099(20)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.‐H. , Strych, U. , Hotez, P. J. , & Bottazzi, M. E. (2020). The SARS‐CoV‐2 vaccine pipeline: An overview. Current Tropical Medicine Reports, 7, 1–4. 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz, M. A. , Chen, Z. , Banach, B. S. , Wang, Y. , Sun, L. , Ratia, K. , & Baker, S. C. (2010). Deubiquitinating and interferon antagonism activities of coronavirus papain‐like proteases. Journal of Virology, 84(9), 4619–4629. 10.1128/jvi.02406-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard, B. , Valle, C. , de Lamballerie, X. , Canard, B. , Seidah, N. G. , & Decroly, E. (2020). The spike glycoprotein of the new coronavirus 2019‐nCoV contains a furin‐like cleavage site absent in CoV of the same clade. Antiviral Research, 176, 104742. 10.1016/j.antiviral.2020.104742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiani, L. , Mancino, E. , Matera, L. , Nenna, R. , Pierangeli, A. , Scagnolari, C. , & Midulla, F. (2020). Will children reveal their secret? The Coronavirus Dilemma. The European Respiratory Journal. 10.1183/13993003.00749-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croston, G. E. (2017). The utility of target‐based discovery. Expert Opinion on Drug Discovery, 12(5), 427–429. 10.1080/17460441.2017.1308351 [DOI] [PubMed] [Google Scholar]
- de Wilde, A. H. , Jochmans, D. , Posthuma, C. C. , Zevenhoven‐Dobbe, J. C. , van Nieuwkoop, S. , Bestebroer, T. M. , … Snijder, E. J. (2014). Screening of an FDA‐approved compound library identifies four small‐molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial Agents and Chemotherapy, 58(8), 4875–4884. 10.1128/aac.03011-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang, L. , Abdelnabi, R. , & Neyts, J. (2018). Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Research, 153, 85–94. 10.1016/j.antiviral.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Deval, J. , Jin, Z. , Chuang, Y. C. , & Kao, C. C. (2017). Structure(s), function(s), and inhibition of the RNA‐dependent RNA polymerase of noroviruses. Virus Research, 234, 21–33. 10.1016/j.virusres.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj, S. G. , Wang, N. , Chen, Z. , Chen, Z. , Tseng, M. , Barretto, N. , & Li, K. (2007). Regulation of IRF‐3‐dependent innate immunity by the papain‐like protease domain of the severe acute respiratory syndrome coronavirus. The Journal of Biological Chemistry, 282(44), 32208–32221. 10.1074/jbc.M704870200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giambenedetto, S. , Ciccullo, A. , Borghetti, A. , Gambassi, G. , Landi, F. , Visconti, E. , & Gasbarrini, A. (2020). Off‐label use of Tocilizumab in patients with SARS‐CoV‐2 infection. Journal of Medical Virology. 10.1002/jmv.25897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, B. , Wang, C. , Tan, Y. , Chen, X. , Liu, Y. , Ning, L. , & Chen, Y. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). medRxiv 2020.2002.2018.20024364. doi: 10.1101/2020.02.18.20024364%J [DOI] [PMC free article] [PubMed]
- Dong, X. , Dong, X. , & Levine, B. (2013). Autophagy and viruses: Adversaries or allies? Journal of Innate Immunity, 5(5), 480–493. 10.1159/000346388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue, M. , Hsieh, F. , Baronas, E. , Godbout, K. , Gosselin, M. , Stagliano, N. , & Acton, S. (2000). A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1‐9. Circulation Research, 87(5), E1–E9. 10.1161/01.res.87.5.e1 [DOI] [PubMed] [Google Scholar]
- Dosch, S. F. , Mahajan, S. D. , & Collins, A. R. (2009). SARS coronavirus spike protein‐induced innate immune response occurs via activation of the NF‐kappaB pathway in human monocyte macrophages in vitro. Virus Research, 142(1–2), 19–27. 10.1016/j.virusres.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, L. , He, Y. , Zhou, Y. , Liu, S. , Zheng, B. J. , & Jiang, S. (2009). The spike protein of SARS‐CoV—A target for vaccine and therapeutic development. Nature Reviews. Microbiology, 7(3), 226–236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder, J. , Sedrani, R. , & Wiesmann, C. (2014). The discovery of first‐in‐class drugs: Origins and evolution. Nature Reviews. Drug Discovery, 13(8), 577–587. 10.1038/nrd4336 [DOI] [PubMed] [Google Scholar]
- Elfiky, A. A. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sciences, 253, 117592. 10.1016/j.lfs.2020.117592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, L. M. (2006). Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Seminars in Oncology, 33(5 Suppl 10), S1–S7. 10.1053/j.seminoncol.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Fan, K. , Wei, P. , Feng, Q. , Chen, S. , Huang, C. , Ma, L. , & Lai, L. (2004). Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C‐like proteinase. The Journal of Biological Chemistry, 279(3), 1637–1642. 10.1074/jbc.M310875200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , & Perlman, S. (2015). Coronaviruses: An overview of their replication and pathogenesis. Methods in Molecular Biology, 1282, 1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore, A. E. , Bridges, C. B. , & Cox, N. J. (2009). Seasonal influenza vaccines. Current Topics in Microbiology and Immunology, 333, 43–82. 10.1007/978-3-540-92165-3_3 [DOI] [PubMed] [Google Scholar]
- Forster, P. , Forster, L. , Renfrew, C. , & Forster, M. (2020). Phylogenetic network analysis of SARS‐CoV‐2 genomes. Proceedings of the National Academy of Sciences of the United States of America, 117, 9241–9243. 10.1073/pnas.2004999117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer, I. H. (2014). Development and implementation of papillomavirus prophylactic vaccines. Journal of Immunology, 192(9), 4007–4011. 10.4049/jimmunol.1490012 [DOI] [PubMed] [Google Scholar]
- Frieman, M. , Heise, M. , & Baric, R. (2008). SARS coronavirus and innate immunity. Virus Research, 133(1), 101–112. 10.1016/j.virusres.2007.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman, M. , Ratia, K. , Johnston, R. E. , Mesecar, A. D. , & Baric, R. S. (2009). Severe acute respiratory syndrome coronavirus papain‐like protease ubiquitin‐like domain and catalytic domain regulate antagonism of IRF3 and NF‐kappaB signaling. Journal of Virology, 83(13), 6689–6705. 10.1128/jvi.02220-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y. , Cheng, Y. , & Wu, Y. (2020). Understanding SARS‐CoV‐2‐mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virologica Sinica. 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta, Y. , Komeno, T. , & Nakamura, T. (2017). Favipiravir (T‐705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 93(7), 449–463. 10.2183/pjab.93.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi, L. , Baehrecke, E. H. , Ballabio, A. , Boya, P. , Bravo‐San Pedro, J. M. , Cecconi, F. , & Kroemer, G. (2017). Molecular definitions of autophagy and related processes. The EMBO Journal, 36(13), 1811–1836. 10.15252/embj.201796697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Tamin, A. , Soloff, A. , D'Aiuto, L. , Nwanegbo, E. , Robbins, P. D. , & Gambotto, A. (2003). Effects of a SARS‐associated coronavirus vaccine in monkeys. Lancet, 362(9399), 1895–1896. 10.1016/s0140-6736(03)14962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Yan, L. , Huang, Y. , Liu, F. , Zhao, Y. , Cao, L. , & Rao, Z. (2020). Structure of the RNA‐dependent RNA polymerase from COVID‐19 virus. Science, 368, 779–782. 10.1126/science.abb7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen, N. C. , Niemeyer, D. , Muth, D. , Corman, V. M. , Martinelli, S. , Gassen, A. , & Rein, T. (2019). SKP2 attenuates autophagy through Beclin1‐ubiquitination and its inhibition reduces MERS‐coronavirus infection. Nature Communications, 10(1), 5770. 10.1038/s41467-019-13659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret, P. , Lagier, J. C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , & Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Glowacka, I. , Bertram, S. , Müller, M. A. , Allen, P. , Soilleux, E. , Pfefferle, S. , & Pöhlmann, S. (2011). Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. Journal of Virology, 85(9), 4122–4134. 10.1128/jvi.02232-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya, A. E. , Baker, S. C. , Baric, R. S. , de Groot, R. J. , Drosten, C. , Gulyaeva, A. A. , & Ziebuhr, J. (2020). Severe acute respiratory syndrome‐related coronavirus: The species and its viruses—A statement of the Coronavirus Study Group. bioRxiv 2020.2002.2007.937862. doi: 10.1101/2020.02.07.937862%J [DOI]
- Gordon, C. J. , Tchesnokov, E. P. , Woolner, E. , Perry, J. K. , Feng, J. Y. , Porter, D. P. , & Gotte, M. (2020). Remdesivir is a direct‐acting antiviral that inhibits RNA‐dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. Journal of Biological Chemistry. 10.1074/jbc.RA120.013679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, D. E. , Jang, G. M. , Bouhaddou, M. , Xu, J. , Obernier, K. , O'Meara, M. J. , & Krogan, N. J. (2020). A SARS‐CoV‐2‐human protein‐protein interaction map reveals drug targets and potential drug‐repurposing. bioRxiv, 2020.2003.2022.002386. doi: 10.1101/2020.03.22.002386%J [DOI]
- Graham, R. L. , Sparks, J. S. , Eckerle, L. D. , Sims, A. C. , & Denison, M. R. (2008). SARS coronavirus replicase proteins in pathogenesis. Virus Research, 133(1), 88–100. 10.1016/j.virusres.2007.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough, T. C. , Babcock, G. J. , Roberts, A. , Hernandez, H. J. , Thomas, W. D., Jr. , Coccia, J. A. , … Ambrosino, D. M. (2005). Development and characterization of a severe acute respiratory syndrome‐associated coronavirus‐neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. The Journal of Infectious Diseases, 191(4), 507–514. 10.1086/427242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti, G. , Raimondi, F. , Ripamonti, D. , Riva, I. , Landi, F. , Alborghetti, L. , & Rambaldi, A. (2020). Use of siltuximab in patients with COVID‐19 pneumonia requiring ventilatory support. medRxiv 2020.2004.2001.20048561. doi: 10.1101/2020.04.01.20048561%J. [DOI]
- Guo, C. , Manjili, M. H. , Subjeck, J. R. , Sarkar, D. , Fisher, P. B. , & Wang, X.‐Y. (2013). Therapeutic cancer vaccines: Past, present, and future. Advances in Cancer Research, 119, 421–475. 10.1016/B978-0-12-407190-2.00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. , Huang, Z. , Lin, L. , & Lv, J. (2020). Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: A viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. Journal of the American Heart Association, 9(7), e016219. 10.1161/jaha.120.016219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, M. T. , Shah, M. N. A. , Paul, S. , Khan, k., & Barua, P. (2020). Zika viral proteome analysis reveals an epitope cluster within NS3 helicase as a potential vaccine candidate: an in silico study. bioRxiv 2020.2004.2024.059543. doi: 10.1101/2020.04.24.059543%J [DOI]
- Harrison, C. (2020). Coronavirus puts drug repurposing on the fast track. Nature Biotechnology, 38(4), 379–381. 10.1038/d41587-020-00003-1 [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, K. V. (2003). SARS‐associated coronavirus. The New England Journal of Medicine, 348(20), 1948–1951. 10.1056/NEJMp030078 [DOI] [PubMed] [Google Scholar]
- Islam, S. M. T. , Zaman, S. , Khan, M. K. , Uddin, M. I. , Chakraborty, S. , Nishat, N. S. , … Seraj, Z. I. (2018). Multi‐Epitope Cluster Ep85B within the Mycobacterial Protein Ag85B Elicits Cell‐Mediated and Humoral Responses in Mice. Turkish Journal of Immunology, 6, 108–117. 10.25002/tji.2018.868 [DOI] [Google Scholar]
- Jiang, S. , Lu, L. , & Du, L. (2013). Development of SARS vaccines and therapeutics is still needed. Future Virology, 8(1), 1–2. 10.2217/fvl.12.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Z. , Du, X. , Xu, Y. , Deng, Y. , Liu, M. , Zhao, Y. , & Yang, H. (2020). Structure of M(pro) from SARS‐CoV‐2 and discovery of its inhibitors. Nature, 582, 289–293. 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- Jin, Z. , Zhao, Y. , Sun, Y. , Zhang, B. , Wang, H. , Wu, Y. , & Rao, Z. (2020). Structural basis for the inhibition of SARS‐CoV‐2 main protease by antineoplastic drug carmofur. Nature Structural & Molecular Biology, 27, 529–532. 10.1038/s41594-020-0440-6 [DOI] [PubMed] [Google Scholar]
- Kadam, R. U. , & Wilson, I. A. (2017). Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proceedings of the National Academy of Sciences of the United States of America, 114(2), 206–214. 10.1073/pnas.1617020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, Y. W. , Okumura, Y. , Kido, H. , Ng, L. F. , Bruzzone, R. , & Altmeyer, R. (2009). Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One, 4(11), e7870. 10.1371/journal.pone.0007870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel, M. , Ibrahim, A. , Fayez, M. , & Al‐Nazawi, M. (2020). From SARS and MERS CoVs to SARS‐CoV‐2: Moving toward more biased codon usage in viral structural and nonstructural genes. Journal of Medical Virology, 92(6), 660–666. 10.1002/jmv.25754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili, J. S. , Zhu, H. , Mak, N. S. A. , Yan, Y. , & Zhu, Y. (2020). Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID‐19. Journal of Medical Virology, 92, 740–746. 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. , Benthin, C. , Zeno, B. , Albertson, T. E. , Boyd, J. , Christie, J. D. , & Lazaar, A. L. (2017). A pilot clinical trial of recombinant human angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Critical Care, 21(1), 234. 10.1186/s13054-017-1823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. K. , Zaman, S. , Chakraborty, S., Chakravorty, R., Alam, M. M., Bhuiyan, T. R., . . . Seraj, Z. I. (2014). In silico predicted mycobacterial epitope elicits in vitro T‐cell responses. Molecular Immunology, 61(1), 16–22. 10.1016/j.molimm.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Khattari, Z. , Brotons, G. , Akkawi, M. , Arbely, E. , Arkin, I. T. , & Salditt, T. (2006). SARS coronavirus E protein in phospholipid bilayers: An x‐ray study. Biophysical Journal, 90(6), 2038–2050. 10.1529/biophysj.105.072892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , Lee, J.‐Y. , Yang, J.‐S. , Kim, J. W. , Kim, V. N. , & Chang, H. (2020). The architecture of SARS‐CoV‐2 transcriptome. bioRxiv 2020.2003.2012.988865. doi: 10.1101/2020.03.12.988865%J [DOI] [PMC free article] [PubMed]
- Kim, E. , Okada, K. , Kenniston, T. , Raj, V. S. , AlHajri, M. M. , Farag, E. A. , & Gambotto, A. (2014). Immunogenicity of an adenoviral‐based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine, 32(45), 5975–5982. 10.1016/j.vaccine.2014.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, U. J. , Won, E. J. , Kee, S. J. , Jung, S. I. , & Jang, H. C. (2016). Combination therapy with lopinavir/ritonavir, ribavirin and interferon‐α for Middle East respiratory syndrome. Antiviral Therapy, 21(5), 455–459. 10.3851/imp3002 [DOI] [PubMed] [Google Scholar]
- Kirchdoerfer, R. N. , & Ward, A. B. (2019). Structure of the SARS‐CoV nsp12 polymerase bound to nsp7 and nsp8 co‐factors. Nature Communications, 10(1), 2342. 10.1038/s41467-019-10280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, H. , Yamada, Y. K. , & Taguchi, F. (1994). Localization of neutralizing epitopes and the receptor‐binding site within the amino‐terminal 330 amino acids of the murine coronavirus spike protein. Journal of Virology, 68(9), 5403–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, L. , Hurst, K. R. , & Masters, P. S. (2007). Exceptional flexibility in the sequence requirements for coronavirus small envelope protein function. Journal of Virology, 81(5), 2249–2262. 10.1128/JVI.01577-06%J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. (2020). Sarah Gilbert: Carving a path towards a COVID‐19 vaccine. Lancet, 395(10232), 1247. 10.1016/s0140-6736(20)30796-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, C. , Fu, W. , Qian, K. , Li, T. , Zhang, S. , Ding, M. , & Hu, S. (2020). Potent neutralization of 2019 novel coronavirus by recombinant ACE2‐Ig. bioRxiv 2020.2002.2001.929976. doi: 10.1101/2020.02.01.929976%J [DOI]
- Lei, J. , Mesters, J. R. , Drosten, C. , Anemüller, S. , Ma, Q. , & Hilgenfeld, R. (2014). Crystal structure of the papain‐like protease of MERS coronavirus reveals unusual, potentially druggable active‐site features. Antiviral Research, 109, 72–82. 10.1016/j.antiviral.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, B. , & Klionsky, D. J. (2004). Development by self‐digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell, 6(4), 463–477. 10.1016/s1534-5807(04)00099-1 [DOI] [PubMed] [Google Scholar]
- Li, F. (2015). Receptor recognition mechanisms of coronaviruses: A decade of structural studies. Journal of Virology, 89(4), 1954–1964. 10.1128/jvi.02615-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. (2016). Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology, 3(1), 237–261. 10.1146/annurev-virology-110615-042301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Berardi, M. , Li, W. , Farzan, M. , Dormitzer, P. R. , & Harrison, S. C. (2006). Conformational states of the severe acute respiratory syndrome coronavirus spike protein ectodomain. Journal of Virology, 80(14), 6794–6800. 10.1128/jvi.02744-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhang, C. , Sui, J. , Kuhn, J. H. , Moore, M. J. , Luo, S. , & Farzan, M. (2005). Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. The EMBO Journal, 24(8), 1634–1643. 10.1038/sj.emboj.7600640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang, T. J. (2009). Varicella zoster virus vaccines: effective, but concerns linger. Canadian Journal of Ophthalmology, 44(4), 379–384. 10.3129/i09-126 [DOI] [PubMed] [Google Scholar]
- Lim, J. , Jeon, S. , Shin, H. Y. , Kim, M. J. , Seong, Y. M. , Lee, W. J. , & Park, S. J. (2020). Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: The application of Lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. Journal of Korean Medical Science, 35(6), e79. 10.3346/jkms.2020.35.e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner, H. A. , Fotouhi‐Ardakani, N. , Lytvyn, V. , Lachance, P. , Sulea, T. , & Ménard, R. (2005). The papain‐like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. Journal of Virology, 79(24), 15199–15208. 10.1128/jvi.79.24.15199-15208.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, J. M. , Heinlein, C. , Kim, T. , Hernandez, S. A. , Malik, M. S. , True, L. D. , & Nelson, P. S. (2014). The androgen‐regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discovery, 4(11), 1310–1325. 10.1158/2159-8290.Cd-13-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung, J. , Lin, Y. S. , Yang, Y. H. , Chou, Y. L. , Shu, L. H. , Cheng, Y. C. , & Wu, C. Y. (2020). The potential chemical structure of anti‐SARS‐CoV‐2 RNA‐dependent RNA polymerase. Journal of Medical Virology, 92, 693–697. 10.1002/jmv.25761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, C. , Zou, L. , Sun, H. , Peng, J. , Gao, C. , Bao, L. , & Sun, S. (2020). A review of the anti‐inflammatory effects of Rosmarinic acid on inflammatory diseases. Frontiers in Pharmacology, 11, 153–153. 10.3389/fphar.2020.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnoli, J. , Narendran, S. , Pereira, F. , Cummings, T. , Hardin, J. W. , Sutton, S. S. , & Ambati, J. (2020). Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19. medRxiv 2020.2004.2016.20065920. doi: 10.1101/2020.04.16.20065920%J [DOI] [PMC free article] [PubMed]
- Marra, M. A. , Jones, S. J. , Astell, C. R. , Holt, R. A. , Brooks‐Wilson, A. , Butterfield, Y. S. , & Roper, R. L. (2003). The genome sequence of the SARS‐associated coronavirus. Science, 300(5624), 1399–1404. 10.1126/science.1085953 [DOI] [PubMed] [Google Scholar]
- Masters, P. S. (2006). The molecular biology of coronaviruses. Advances in Virus Research, 66, 193–292. 10.1016/s0065-3527(06)66005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama, S. , Nao, N. , Shirato, K. , Kawase, M. , Saito, S. , Takayama, I. , & Takeda, M. (2020). Enhanced isolation of SARS‐CoV‐2 by TMPRSS2‐expressing cells. Proceedings of the National Academy of Sciences of the United States of America, 117(13), 7001–7003. 10.1073/pnas.2002589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra, M. R. , Desai, S. S. , Ruschitzka, F. , & Patel, A. N. (2020). Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: A multinational registry analysis. Lancet. 10.1016/s0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Molina, J. M. , Delaugerre, C. , Le Goff, J. , Mela‐Lima, B. , Ponscarme, D. , Goldwirt, L. , & de Castro, N. (2020). No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Médecine et Maladies Infectieuses, 50, 384. 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil, V. , Kwon, H. , Prado, P. , Hagelkrüys, A. , Wimmer, R. A. , Stahl, M. , & Penninger, J. M. (2020). Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell, 181, 905–913.e7. 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, J. S. , Lalonde, T. , Xu, S. , & Liu, W. R. (2020). Learning from the past: Possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. ChemBioChem, 21(5), 730–738. 10.1002/cbic.202000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Project for SARS (NSPS) . (2004). The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. (2004). American Journal of Clinical Pathology, 121(4), 507–511. 10.1309/wpk7-y2xk-nf4c-bf3r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich‐Zugich, J. , Knox, K. S. , Rios, C. T. , Natt, B. , Bhattacharya, D. , & Fain, M. J. (2020). SARS‐CoV‐2 and COVID‐19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience, 42, 1–10. 10.1007/s11357-020-00186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, X. , Liu, Y. , Lei, X. , Li, P. , Mi, D. , Ren, L. , & Qian, Z. (2020). Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nature Communications, 11(1), 1620. 10.1038/s41467-020-15562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, S. F. , & Ho, Y. C. (2020). SARS‐CoV‐2: A storm is raging. The Journal of Clinical Investigation, 130, 2202–2205. 10.1172/jci137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Martínez, A. , Valentín, J. , Fernández, L. , Hernández‐Jiménez, E. , López‐Collazo, E. , Zerbes, P. , & Pfeiffer, M. M. (2015). Arabinoxylan rice bran (MGN‐3/biobran) enhances natural killer cell‐mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy, 17(5), 601–612. 10.1016/j.jcyt.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Pervushin, K. , Tan, E. , Parthasarathy, K. , Lin, X. , Jiang, F. L. , Yu, D. , & Torres, J. (2009). Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathogens, 5(7), e1000511. 10.1371/journal.ppat.1000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar, T. , Manickam, M. , Namasivayam, V. , Hayashi, Y. , & Jung, S. H. (2016). An overview of severe acute respiratory syndrome‐coronavirus (SARS‐CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy. Journal of Medicinal Chemistry, 59(14), 6595–6628. 10.1021/acs.jmedchem.5b01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia, K. , Saikatendu, K. S. , Santarsiero, B. D. , Barretto, N. , Baker, S. C. , Stevens, R. C. , & Mesecar, A. D. (2006). Severe acute respiratory syndrome coronavirus papain‐like protease: Structure of a viral deubiquitinating enzyme. Proceedings of the National Academy of Sciences of the United States of America, 103(15), 5717–5722. 10.1073/pnas.0510851103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimanshee, A. , Amit, D. , Vishal, P. , & Mukesh, K. (2020). Potential inhibitors against papain‐like protease of novel coronavirus (SARS‐CoV‐2) from FDA approved drugs. ChemRxiv.
- Roberts, A. , Thomas, W. D. , Guarner, J. , Lamirande, E. W. , Babcock, G. J. , Greenough, T. C. , & Ambrosino, D. M. (2006). Therapy with a severe acute respiratory syndrome‐associated coronavirus‐neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. The Journal of Infectious Diseases, 193(5), 685–692. 10.1086/500143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, C. D. , Millar, J. E. , & Baillie, J. K. (2020). Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet, 395(10223), 473–475. 10.1016/s0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma, P. , Kaur, H. , Kumar, H. , Mahendru, D. , Avti, P. , Bhattacharyya, A. , & Medhi, B. (2020). Virological and clinical cure in COVID‐19 patients treated with hydroxychloroquine: A systematic review and meta‐analysis. Journal of Medical Virology, 92(7), 776–785. 10.1002/jmv.25898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek, S. , Shah, R. , & Munshi, N. C. (2016). Review of siltuximab in the treatment of multicentric Castleman's disease. Therapeutic Advances in Hematology, 7(6), 360–366. 10.1177/2040620716653745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino, A. , Boelaert, J. R. , Cassone, A. , Majori, G. , & Cauda, R. (2003). Effects of chloroquine on viral infections: An old drug against today's diseases? The Lancet Infectious Diseases, 3(11), 722–727. 10.1016/s1473-3099(03)00806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybert, A. , Posthuma, C. C. , van Dinten, L. C. , Snijder, E. J. , Gorbalenya, A. E. , & Ziebuhr, J. (2005). A complex zinc finger controls the enzymatic activities of nidovirus helicases. Journal of Virology, 79(2), 696–704. 10.1128/jvi.79.2.696-704.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, M. N. , Barua, P. , & Khan, M. K (2015). Immunoinformatics aided prediction of cytotoxic T cell epitope of respiratory syncytial virus. Bioresearch Communications (BRC), 1(2), 99–104. [Google Scholar]
- Shang, L. , Zhao, J. , Hu, Y. , Du, R. , & Cao, B. (2020). On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet, 395(10225), 683–684. 10.1016/s0140-6736(20)30361-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T. P. , Sims, A. C. , Leist, S. R. , Schäfer, A. , Won, J. , Brown, A. J. , & Baric, R. S. (2020). Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nature Communications, 11(1), 222. 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T. P. , Sims, A. C. , Zhou, S. , Graham, R. L. , Pruijssers, A. J. , Agostini, M. L. , … Baric, R. S. (2020). An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Science Translational Medicine, 12(541), eabb5883. 10.1126/scitranslmed.abb5883%J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C. , Wang, Z. , Zhao, F. , Yang, Y. , Li, J. , Yuan, J. , & Liu, L. (2020). Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. Journal of the American Medical Association, 323, 1582. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla, A. , Heald‐Sargent, T. , Subramanya, G. , Zhao, J. , Perlman, S. , & Gallagher, T. (2011). A Transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. Journal of Virology, 85(2), 873–882. 10.1128/JVI.02062-10%J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum, K. T. , & Tanner, J. A. (2008). Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. ChemBioChem, 9(18), 3037–3045. 10.1002/cbic.200800491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, D. , Hui, H. C. , Doerffler, E. , Clarke, M. O. , Chun, K. , Zhang, L. , & Mackman, R. L. (2017). Discovery and synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1‐f][triazin‐4‐amino] adenine C‐nucleoside (GS‐5734) for the treatment of Ebola and emerging viruses. Journal of Medicinal Chemistry, 60(5), 1648–1661. 10.1021/acs.jmedchem.6b01594 [DOI] [PubMed] [Google Scholar]
- Simmons, G. , Reeves, J. D. , Rennekamp, A. J. , Amberg, S. M. , Piefer, A. J. , & Bates, P. (2004). Characterization of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) spike glycoprotein‐mediated viral entry. Proceedings of the National Academy of Sciences of the United States of America, 101(12), 4240–4245. 10.1073/pnas.0306446101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, Y. L. , Teoh, K. T. , Lo, J. , Chan, C. M. , Kien, F. , Escriou, N. , & Nal, B. (2008). The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus‐like particles. Journal of Virology, 82(22), 11318–11330. 10.1128/jvi.01052-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola, I. , Almazán, F. , Zúñiga, S. , & Enjuanes, L. (2015). Continuous and discontinuous RNA synthesis in coronaviruses. Annual Review of Virology, 2(1), 265–288. 10.1146/annurev-virology-100114-055218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, P. , & Lin, S. X. (2018). RNA‐dependent RNA polymerase: Addressing Zika outbreak by a phylogeny‐based drug target study. Chemical Biology & Drug Design, 91(1), 322–327. 10.1111/cbdd.13054 [DOI] [PubMed] [Google Scholar]
- Sterner, R. M. , Sakemura, R. , Cox, M. J. , Yang, N. , Khadka, R. H. , Forsman, C. L. , & Kenderian, S. S. (2019). GM‐CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR‐T cell function in xenografts. Blood, 133(7), 697–709. 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz, S. , Reichelt, M. , Spiegel, M. , Kuri, T. , Martínez‐Sobrido, L. , García‐Sastre, A. , & Kochs, G. (2007). The intracellular sites of early replication and budding of SARS‐coronavirus. Virology, 361(2), 304–315. 10.1016/j.virol.2006.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subissi, L. , Imbert, I. , Ferron, F. , Collet, A. , Coutard, B. , Decroly, E. , & Canard, B. (2014). SARS‐CoV ORF1b‐encoded nonstructural proteins 12‐16: Replicative enzymes as antiviral targets. Antiviral Research, 101, 122–130. 10.1016/j.antiviral.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, W. , He, L. , Zhang, X. , Pu, J. , Voronin, D. , Jiang, S. , & Du, L. (2020). Characterization of the receptor‐binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & Molecular Immunology, 17, 613–620. 10.1038/s41423-020-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, R. C. , Lau, J. Y. N. , & Hong, Z. (2001). Mechanisms of action of ribavirin in antiviral therapies. Antiviral Chemistry and Chemotherapy, 12(5), 261–272. 10.1177/095632020101200501 [DOI] [PubMed] [Google Scholar]
- Tan, E. L. , Ooi, E. E. , Lin, C. Y. , Tan, H. C. , Ling, A. E. , Lim, B. , & Stanton, L. W. (2004). Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerging Infectious Diseases, 10(4), 581–586. 10.3201/eid1004.030458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner, J. A. , Watt, R. M. , Chai, Y. B. , Lu, L. Y. , Lin, M. C. , Peiris, J. S. , & Huang, J. D. (2003). The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases. The Journal of Biological Chemistry, 278(41), 39578–39582. 10.1074/jbc.C300328200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokov, E. P. , Feng, J. Y. , Porter, D. P. , & Götte, M. (2019). Mechanism of inhibition of Ebola virus RNA‐dependent RNA polymerase by Remdesivir. Viruses, 11(4), 326. 10.3390/v11040326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira da Silva, J. A. (2020). Convalescent plasma: A possible treatment of COVID‐19 in India. Medical Journal, Armed Forces India, 76, 236–237. 10.1016/j.mjafi.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X. , Li, C. , Huang, A. , Xia, S. , Lu, S. , Shi, Z. , & Ying, T. (2020). Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus‐specific human monoclonal antibody. Emerging Microbes & Infections, 9(1), 382–385. 10.1080/22221751.2020.1729069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik, J. R. , Korth, M. J. , Simmons, C. P. , Farrar, J. , Martin, T. R. , & Katze, M. G. (2012). Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews, 76(1), 16–32. 10.1128/mmbr.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici, M. A. , Walls, A. C. , Lang, Y. , Wang, C. , Li, Z. , Koerhuis, D. , & Veesler, D. (2019). Structural basis for human coronavirus attachment to sialic acid receptors. Nature Structural & Molecular Biology, 26(6), 481–489. 10.1038/s41594-019-0233-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp, R. A. , Haynes, L. M. , Moore, D. , Anderson, B. , Tamin, A. , Harcourt, B. H. , & Anderson, L. J. (2005). Monoclonal antibodies to SARS‐associated coronavirus (SARS‐CoV): Identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. Journal of Virological Methods, 128(1–2), 21–28. 10.1016/j.jviromet.2005.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkov, T. , Carbone, V. , Akter, J. , Sivanesan, S. , Li, J. , Beddoe, T. , & Marsh, G. A. (2014). The RNA‐dependent‐RNA polymerase, an emerging antiviral drug target for the Hendra virus. Current Drug Targets, 15(1), 103–113. 10.2174/1389450114888131204163210 [DOI] [PubMed] [Google Scholar]
- Venkataraman, S. , Prasad, B. , & Selvarajan, R. (2018). RNA dependent RNA polymerases: Insights from structure, function and evolution. Viruses, 10(2). 10.3390/v10020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong, W. , Khan, M. B. , Fischer, C. , Arutyunova, E. , Lamer, T. , Shields, J. , & Lemieux, J. (2020). Feline coronavirus drug inhibits the main protease of SARS‐CoV‐2 and blocks virus replication. bioRxiv. [DOI] [PMC free article] [PubMed]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292.e286. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS coronavirus. Journal of Virology, 94(7). 10.1128/jvi.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , & Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Research, 30(3), 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. Z. , Cai, B. Q. , Li, L. Y. , Lin, J. T. , Su, N. , Yu, H. X. , & Liu, L. (2004). Efficacy and safety of arbidol in treatment of naturally acquired influenza. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 26(3), 289–293. [PubMed] [Google Scholar]
- Wang, N. , Shi, X. , Jiang, L. , Zhang, S. , Wang, D. , Tong, P. , & Wang, X. (2013). Structure of MERS‐CoV spike receptor‐binding domain complexed with human receptor DPP4. Cell Research, 23(8), 986–993. 10.1038/cr.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, D. , Du, G. , Du, R. , Zhao, J. , Jin, Y. , & Wang, C. (2020). Remdesivir in adults with severe COVID‐19: A randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet, 395, 1569–1578. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, L. , McKinlay, C. , Gage, P. , & Ewart, G. (2004). SARS coronavirus E protein forms cation‐selective ion channels. Virology, 330(1), 322–331. 10.1016/j.virol.2004.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S. K. , Li, W. , Moore, M. J. , Choe, H. , & Farzan, M. (2004). A 193‐amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin‐converting enzyme 2. The Journal of Biological Chemistry, 279(5), 3197–3201. 10.1074/jbc.C300520200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp, D. , Wang, N. , Corbett, K. S. , Goldsmith, J. A. , Hsieh, C. L. , Abiona, O. , & McLellan, J. S. (2020). Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science, 367(6483), 1260–1263. 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Liu, Y. , Yang, Y. , Zhang, P. , Zhong, W. , Wang, Y. , & Li, H. (2020). Analysis of therapeutic targets for SARS‐CoV‐2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B, 10, 766–788. 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Xie, W. , Xue, X. , Yang, K. , Ma, J. , Liang, W. , & Rao, Z. (2005). Design of wide‐spectrum inhibitors targeting coronavirus main proteases. PLoS Biology, 3(10), e324. 10.1371/journal.pbio.0030324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N. , & Shen, H. M. (2020). Targeting the Endocytic pathway and autophagy process as a novel therapeutic strategy in COVID‐19. International Journal of Biological Sciences, 16(10), 1724–1731. 10.7150/ijbs.45498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. , Ye, F. , Zhang, M. , Cui, C. , Huang, B. , Niu, P. , & Liu, D. (2020). In vitro antiviral activity and projection of optimized dosing Design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clinical Infectious Diseases. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong, C. Y. , Ong, H. K. , Yeap, S. K. , Ho, K. L. , & Tan, W. S. (2019). Recent advances in the vaccine development against Middle East respiratory syndrome‐coronavirus. Frontiers in Microbiology, 10, 1781–1781. 10.3389/fmicb.2019.01781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Wada, J. , Hida, K. , Tsuchiyama, Y. , Hiragushi, K. , Shikata, K. , & Makino, H. (2001). Collectrin, a collecting duct‐specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. The Journal of Biological Chemistry, 276(20), 17132–17139. 10.1074/jbc.M006723200 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Lin, D. , Sun, X. , Curth, U. , Drosten, C. , Sauerhering, L. , & Hilgenfeld, R. (2020). Crystal structure of SARS‐CoV‐2 main protease provides a basis for design of improved α‐ketoamide inhibitors. Science, 368, 409–412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Wu, Q. , & Zhang, Z. (2020). Probable pangolin origin of SARS‐CoV‐2 associated with the COVID‐19 outbreak. Current Biology, 30(7), 1346–1351.e1342. 10.1016/j.cub.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, W. , Thorne, N. , & McKew, J. C. (2013). Phenotypic screens as a renewed approach for drug discovery. Drug Discovery Today, 18(21–22), 1067–1073. 10.1016/j.drudis.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Fu, B. , Zheng, X. , Wang, D. , Zhao, C. , Qi, Y. , & Wei, H. (2020). Aberrant pathogenic GM‐CSF T cells and inflammatory CD14 monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv 2020.2002.2012.945576. doi: 10.1101/2020.02.12.945576%J [DOI]
- Zhu, J.‐D. , Meng, W. , Wang, X.‐J. , & Wang, H.‐C. R. (2015). Broad‐spectrum antiviral agents. Frontiers in Microbiology, 6, 517–517. 10.3389/fmicb.2015.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla, A. , Chan, J. F. , Azhar, E. I. , Hui, D. S. , & Yuen, K. Y. (2016). Coronaviruses ‐ drug discovery and therapeutic options. Nature Reviews. Drug Discovery, 15(5), 327–347. 10.1038/nrd.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]


