Abstract
The rapid spread of coronavirus disease 2019 (COVID‐19) represented the most serious issue to public health globally. Hematological patients as immunocompromised hosts are vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. There is little information available regarding the clinical features of hematological patients concomitant with COVID‐19. In this study, 9 concomitant patients were analyzed for their clinical manifestations, laboratory data, radiological findings, and immunologic features. The median age was 50 years (range, 17‐68 years) and 6 patients were male. Seven patients were infected through hospital‐associated transmission and other 2 through community‐associated transmission. Onset of COVID‐19 in all patients occurred during routine treatments for their hematological diseases. Eight patients were classified as moderate and 1 patient as critically ill COVID‐19. Four patients died, 1 from leukemia progression, 2 from life‐threatening secondary infection, and the other from respiratory failure caused by COVID‐19. Abruptly elevated levels of cytokines were often correlated with progressive hematological disease or concurrent bacterial infections. Two patients had atypical computed tomography (CT) imaging findings of COVID‐19. The median interval from the first CT scan imaging to improvement in survivors was 40 days (range, 14‐51 days). Four of 5 survivors had negative serological tests 1 month after symptom onset. Positive viral load in 4 survivors lasted longer than 45 days. Our results indicated concomitant patients formed a distinct subgroup characterized by atypical clinical features, defective viral clearance, and lower level of SARS‐CoV‐2‐specific Abs. Targeted therapies that impair host humoral immunity should be avoided. These findings will be helpful to tailor appropriate management for the concomitant patients.
Keywords: COVID‐19, hematological patient, immunocompromised state, SARS‐CoV‐2
Hematological patients concomitant with COVID‐19 formed a distinct subgroup with atypical clinical features. Given the importance of humoral immunity during viral clearance, targeted agents that impair host humoral immunity should be avoided.
Abbreviations
- Abs
antibodies
- B‐ALL
acute B lymphoblastic leukemia
- BCMA‐CAR
B‐cell maturation antigen chimeric antigen receptor
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- ddPCR
droplet digital PCR
- GGO
ground glass opacity
- IL‐6
interleukin‐6
- i.v.
intravenous
- MDR
multiple drug‐resistant
- MGB
minor groove binder
- MM
multiple myeloma
- PCT
procalcitonin
- RT‐PCR
real‐time reverse‐transcriptase polymerase‐chain‐reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TKI
tyrosine kinase inhibitor
- WHO
World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 and the disease it causes, COVID‐19, are an emerging public health threat. 1 Since the first case was identified in early December 2019 in Wuhan city, 2 more than 80 000 cases of COVID‐19 have been confirmed in China. Given its rapid global spread, COVID‐19 has now been declared a pandemic by the WHO. 3
Knowledge of the disease epidemiology, clinical presentation, and immunological features has been constantly evolving. 4 , 5 , 6 Given that there is still no vaccine or specific antiviral treatment, once the SARS‐CoV‐2 infects the body, virus clearance depends on adaptive immunity. Hematological patients make up a highly vulnerable population characterized by impaired immunity due to immune suppression or anticancer therapy and are at higher risk of developing infections. To date, there is little information available regarding the hematological patients concomitant with COVID‐19. The clinical features and appropriate management of these immunocompromised patients remain unclear. There is an urgent unmet medical need for hematologists to collect more information regarding SARS‐CoV‐2 infection in hematological patients, which needs to be solved immediately. Recently, Jin and colleagues described a case of a patient with chronic lymphocytic leukemia with COVID‐19. 7 This patient had an atypical clinical course and biochemical data with a longer incubation period and predominantly elevated lymphocyte count. However, many critical issues for SARS‐Cov‐2 infected hematological patients remain elusive and many more clinical studies should be carried out.
In the study, we retrospectively analyzed the clinical characteristics and laboratory features of 9 concomitant patients, which could assist hematologists to tailor appropriate treatments for improved clinical outcomes.
2. MATERIALS AND METHODS
2.1. Data sources
A total of 9 patients with hematological disorders concomitant with SARS‐CoV‐2 infection from an isolation ward of Tongji Hospital (Wuhan, Hubei Province, China), were examined between 1 and 29 February 2020. Tongji Hospital is one of the major hospitals assigned by the government for the treatment of SARS‐CoV‐2 infection. The criteria and definitions for diagnosis, clinical classification (mild, moderate, severe, and critically ill) and discharge standards were based on Guidance for Corona Virus Disease 2019. 8 The clinical outcomes were monitored up to 31 March 2020.
The Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ‐C20200114) approved the study. Written informed consent was obtained from all patients.
2.2. Determination of SARS‐CoV‐2
Throat swab specimens were collected for extracting SARS‐CoV‐2 RNA. The RT‐PCR assay protocol for SARS‐CoV‐2 was based on the recommendation by the National Institute for Viral Disease Control and Prevention (China). 9
Plasma samples were collected for extracting SARS‐CoV‐2 RNA. Viral RNA purification kit (QIAamp Viral RNA Mini Kit; Qiagen), one‐step RT‐ddPCR advanced kit, QX200 droplet generator (Bio‐Rad), and QX200 droplet reader (Bio‐Rad) were used for quantitative detection of SARS‐CoV‐2 copy numbers, following the manufacturer’s instructions.
To increase sensitivity, a 4‐well test was carried out per sample in this study. The SARS‐CoV‐2‐specific MGB probe‐primer set was designed for targeting the orf1ab region, and the sequences were as follows: forward primer, 5′‐TGACCCTGTGGGTTTTACACTTAA‐3′; reverse primer, 5′CAGCCATAACCTTTCCACATACC3′; and probe, 5′‐FAM‐AACACAGTCTGTACCGTCT‐MGB‐3′.
2.3. Serological determination of SARS‐CoV‐2‐specific IgM and IgG
Serological testing for COVID‐19 was carried out with paramagnetic particle chemiluminescent immunoassay using an iFlash‐SARS‐CoV‐2 IgM/IgG assay kit (Shenzhen Yhlo Biotech) and iFlash Immunoassay Analyzer (Shenzhen Yhlo Biotech)
3. RESULTS
3.1. Patient characteristics
During the period 1 February 2020 to 29 February 2020, 9 hematological patients were diagnosed with COVID‐19 in hematological wards (7 patients) or fever clinics (2 patients) and transferred to isolation wards of Tongji Hospital. The nosocomial infection prevalence rate of inpatients in the hematological department of Tongji hospital was 2.8%. The median age was 50 years (range, 17‐68 years) and 6 patients were male. Among the 9 patients, there was 1 with aplastic anemia, 3 with acute myeloid leukemia, 2 with Ph+ B‐ALL, 1 with relapsed Ph‐like B‐ALL, 1 with diffuse large B‐cell lymphoma, and 1 with MM. At the time of diagnosis of COVID‐19, all 9 patients were in their active treatments: 8 of 9 patients had received either i.v. chemotherapy and/or oral drugs (dasatinib, sorafenib, venetoclax, and cyclosporine A); another patient with refractory/relapsed MM was in complete remission, but had persistent B cell and plasma cell aplasia following BCMA‐CAR T cell therapy 3 months previously. The routine treatments for hematological disorders were adjusted for all patients, including delayed treatments in 4 patients, suspended therapy in 3 patients, and reduced‐dosage chemotherapy in 2 patients (dasatinib 100 mg/d and methylprednisolone 40 mg/d). Of note, at the time they were transferred to isolation wards, 3 (33.3%) patients had progressive primary diseases with neutropenia (Table 1).
TABLE 1.
Clinical characteristics and treatment of 9 hematological patients with COVID‐19
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 53 | 57 | 45 | 64 | 31 | 17 | 30 | 68 | 50 |
| Sex | Female | Male | Male | Male | Male | Male | Female | Female | Male |
| Hematological disease | |||||||||
| Diagnosis | AA | AML‐M5 | AML‐M5 | AML‐M5 | Ph+ B‐ALL | Ph+ B‐ALL | Ph‐like ALL | DLBCL | MM |
| Remission | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Agranulocytosis | Yes | No | No | Yes | No | No | Yes | No | No |
| Time of last treatment | Within 1 mo | Within 1 mo | Within 1 mo | 4 d ago | Duration | Within 1 mo | Duration | Within 1 mo | Within 3 mo |
| Last treatment regimen | CsA | Dec, HA | Dec, IA | Dec | Dasatinib | Dasatinib, VP | Sorafenib Venetoclax | R‐CHOP | BCMA‐CAR T cell |
| SARS‐CoV‐2 | |||||||||
| Associated transmission | Community | Hospital | Hospital | Hospital | Hospital | Hospital | Hospital | Community | Hospital |
| Clinical manifestation | |||||||||
| Fever | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Fatigue | Yes | Yes | No | Yes | No | No | Yes | No | No |
| Dry cough | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Expectoration | No | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Hemoptysis | Yes | No | Yes | No | No | No | No | No | No |
| Dyspnea | Yes | No | Yes | No | No | No | No | Yes | Yes |
| Diarrhea | No | No | No | No | Yes | No | Yes | No | No |
| Nausea and vomiting | No | No | No | No | No | No | Yes | No | No |
| Clinical classification | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Critically |
| Nucleic acid and Ab testing | |||||||||
| Throat swabs | Negative | Positive | Negative | Positive | Negative | Negative | Positive | Positive | Positive |
| Plasma (copies/mL) | ND | ND | 1384 | ND | 250 | 116 | ND | ND | 5044 |
| Ab | Positive | Positive | Negative | ND | Negative | Negative | ND | Negative | Negative |
| Therapy | |||||||||
| Antiviral | Arbidol | Arbidol Lopinavir Ritonavir | Arbidol | Arbidol | Arbidol Lopinavir Ritonavir | Arbidol Lopinavir Ritonavir | Arbidol Lopinavir Ritonavir | Arbidol | Arbidol Lopinavir Ritonavir |
| Antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Antifungal | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| Immunoglobulin | Yes | Yes | No | Yes | No | No | Yes | No | Yes |
| Corticosteroids | No | No | No | No | Yes | Yes | No | No | Yes |
| Oxygen therapy | IMV | No | Nasal catheter | NMV | Nasal catheter |
Nasal catheter |
Nasal catheter |
Nasal catheter | NMV |
| Clinical outcomes | |||||||||
| Outcome | Death | Stay in hospital | Discharged from hospital | Death | Discharged from hospital |
Discharged from hospital |
Death | Discharged from hospital | Death |
| Cause of death | Septicopyemia | NA | NA | Septic shock | NA | NA | Intracranial invasion | NA | Respiratory failure |
Abbreviations: Ab, antibody; AA, aplastic anemia; AML‐M5, acute monocytic leukemia; B‐ALL, acute B lymphoblastic leukemia; BCMA‐CAR, B‐cell maturation antigen chimeric antigen receptor; CsA, cyclosporine A; Dec, decitabine; DLBCL, diffuse large B‐cell lymphoma; HA, homoharringtonine, cytarabine; IA, idarubicin, cytarabine; IMV, invasive mechanical ventilation; MM, multiple myeloma; NA, not available; ND, not done; NMV, noninvasive mechanical ventilation; R‐CHOP, rituximab, cyclophosphamide, epirubicin, vindesine, prednisolone; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; VP, vindesine, prednisolone.
The most common onset symptoms in this case series were dry cough (8/9, 88.9%), expectoration (5/9, 55.6%), and fever (6/9, 66.7%). Other symptoms included 4 patients (44.4%) with fatigue, 3 (33.3%) with dyspnea, 2 (22.2%) with diarrhea, 2 (22.2%) with hemoptysis, and 1 (11.1%) with vomiting. Eight (88.9%) patients were classified as moderately ill, and 1 (11.1%) was critically ill. All patients received standard supportive care, including antiviral therapy (arbidol, 9 [100%]; lopinavir/ritonavir, 5 [55.6%]), oxygen therapy, 5 [55.6%]), mechanical ventilation (noninvasive, 2 [22.2%]; invasive, 1 [11.1%]), i.v. antibiotics (8 [88.9%]) and antifungal therapy (7 [77.8%]). Only 3 (33.3%) cases received systemic corticosteroids therapy. At the final date of follow‐up, 4 patients had been discharged after meeting the discharge criteria 8 and 1 patient was still in the hospital for treatment. Four (44.4%) patients died (2 died of severe bacterial infection, 1 of leukemia progression, and 1 of respiratory failure caused by COVID‐19). Of note, both Ph+ ALL patients concomitant with COVID‐19 had been discharged without leukemia progression.
3.2. Inflammatory indicators in SARS‐CoV‐2‐infected hematological patients
All cases had elevated levels of inflammatory factors (Figure 1A); however, the causes of elevated inflammatory markers were varied. Significantly, with the resolution of COVID‐19‐related symptoms, extremely elevated levels of CRP (greater than 100 [normal value less than 1 mg/L]), PCT (greater than 50, [normal value less than 0.05 ng/L]), and IL‐6 (greater than 5000 [normal value less than 7 pg/mL]) occurred in 3, 2, and 2 cases, respectively, which was proved to be caused by secondary infections. Strikingly elevated levels of ferritin (greater than 10 000 [normal range, 30‐400 μg/L]) were detected in 2 cases, of which, 1 case underwent leukemia progression and the other experienced deterioration of COVID‐19. Three representative cases were depicted in Figure 1B‐E. The moderate COVID‐19 patient (case 2) had declined inflammation factors with resolution of COVID‐19. The critically ill patient (case 9) had much higher inflammation factors than those in case 2. In case 1, CRP, PCT, and IL‐6 levels decreased with the clinical improvement of COVID‐19 in the beginning. However, fever was present again on day 13, accompanied by an abrupt increase in CRP, PCT, and IL‐6 levels (Figure 1B‐D, black arrow), caused by MDR Acinetobacter baumannii septicemia.
FIGURE 1.
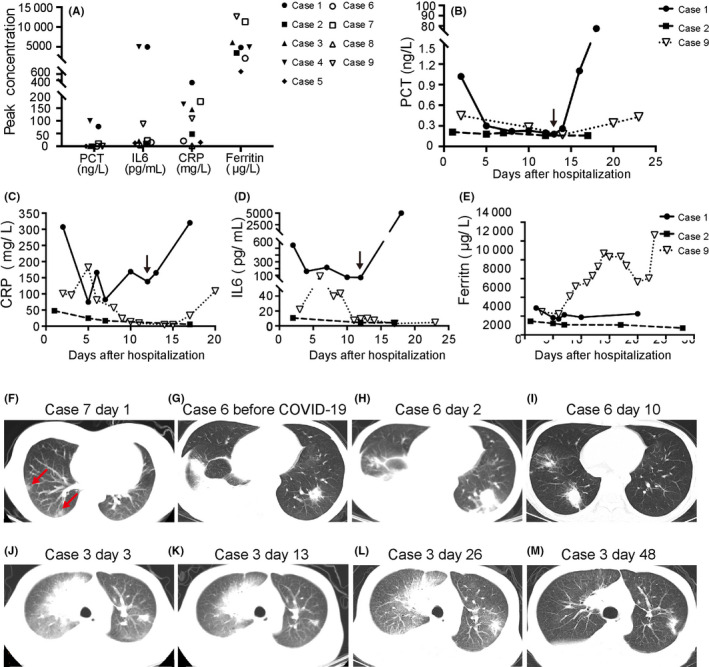
Levels of inflammatory markers in hematological patients infected with severe acute respiratory syndrome coronavirus 2 and features of chest computed tomography (CT). A, Peak concentrations of inflammatory markers in 9 cases. B‐E, Time course of procalcitonin (PCT) (B), C‐reactive protein (CRP) (C), interleukin‐6 (IL‐6) (D), and ferritin (E) levels in cases 1, 2, and 9. F, CT image from Case 7 shows nodules in the subpleural areas on day 1 after symptom onset. G‐I, Axial images from Case 6 showed bilateral patchy consolidations and pleural effusion before (G) and on day 2 after confirmation of COVID‐19 infection (H), and improvement on day 10 (I). J‐M, CT images from Case 3 showed mixed ground glass opacity with consolidation and a single side pleural effusion on day 3 (J), 13 (K), and 26 (L) after symptom onset, and improvement on day 48 (M)
3.3. Chest CT manifestation
All patients had abnormal CT imaging findings on admission. Of the 9 cases, 7 (77.8%) patients had the typical imaging findings of COVID‐19, namely GGO or mixed GGO with consolidation; 2 patients had nodules in the subpleural areas (Figure 1F, red arrow) or patchy bilateral consolidations (Figure 1H) but no typical imaging findings of COVID‐19. Among the 9 patients, 3 patients had other respiratory infection before COVID‐19, according to symptoms and abnormal image appearances, which complicated CT imaging findings. The preexisting abnormal image appearances included patchy lesions, nodules, and pleural effusion. Two of these 3 patients had new advanced GGO lesions, and the other had progression of original lesions and an increase of pleural effusion without typical imaging features of COVID‐19 (Figure 1G‐I).
A follow‐up chest CT scan was carried out 7‐14 days after admission in 8 patients, which showed improvement in 2 patients (25%), unchanged appearance in 5 patients (62.5%), and deterioration in 1 patient (12.5%). Follow‐up scans were not available for the patient who died in the course of study. Notably, the median interval from the first CT scan imaging after COVID‐19 confirmation to improvement was 40 days (range, 14‐51 days, n = 5). Subsequent imaging data were not obtained for the other 4 patients due to death. Patient 3 had typical imaging findings at initial chest CT (Figure 1J), unchanged appearance at the second and third follow‐up scans (Figure 1K,L), and improved image at 48 days after diagnosis of the disease (Figure 1M).
3.4. Immunologic features of 6 patients and duration of SARS‐CoV‐2
Immunologic features of 6 patients in hematological remission state were analyzed. Preliminary analysis of circulating immune cell subsets showed that an absolute number of both CD8+ T cells and B cells were below the normal value in all 4 tested cases. In particular, the complete absence of B cells occurred in 2 patients who had received immunotherapy (rituximab or CAR T‐cell therapy). Serological testing showed detectable anti‐SARS‐CoV‐2 Abs 1 month after onset of symptoms in only 1 of 6 patients (case 2). Of 4 discharged patients, 3 still tested positive for SARS‐CoV‐2 in plasma. In 4 of 5 survivors, nucleic acid testing for SARS‐CoV‐2 remained positive for at least 45 days. The average length of hospitalization of the 5 survivors was over 39.6 days; 1 patient is still in hospital due to significant delay in viral clearance as detected by swab test (Table 2).
TABLE 2.
Immune state of 6 hematological patients and duration of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection
| Clinical outcome | Circulating immune cell subsets a | Anti‐SARS‐CoV‐2 Abs (normal value < 10 AU/mL) | Duration of SARS‐CoV‐2 infection (d) b | Total course in hospital (d) c | |||||
|---|---|---|---|---|---|---|---|---|---|
| B cells (/μL) | T cells (/μL) | IgM (AU/mL) | IgG (AU/mL) | Detection time point (day after illness onset) | |||||
| CD4+ T cells | CD8+ T cells | ||||||||
| Alive | Case 2 | 46 | 66 | 101 | 16.53 | 65.08 | 32 | 48+ | 48+ |
| Case 3 | 41 | 719 | 284 | 0.94 | 1.42 | 33 | 48+ | 48 | |
| Case 5 | ND | ND | ND | 1.76 | 0.76 | 18 | 45+ | 23 | |
| Case 6 | ND | ND | ND | 7.31 | 2.74 | 21 | 53+ | 27 | |
| Case 8 | 1 | 257 | 234 | 1.37 | 1.26 | 38 | 19 | 52 | |
| Dead | Case 9 | 0 | 37 | 119 | 0.48 | 2.97 | 23 | 23 | 23 |
Abbreviation: Abs, antibodies; ddPCR, droplet digital PCR; ND, not done.
Normal range of circulating immune cell subsets: B cells, 90‐560/μL; helper T cells, 550‐1440/μL; suppressor/cytotoxic T cells, 320‐1250/μL.
Determination of plasma nucleic acid of SARS‐CoV‐2 in cases 3, 5, and 6 by One‐step RT‐ddPCR, SARS‐CoV‐2 infection as confirmed by the positive result of a throat swab test in cases 2 and 8. +, SARS‐CoV‐2 infection continued.
+, Case 2 is still in hospital.
4. DISCUSSION
Our results illustrated the following clinical characteristics. First, patients with hematological disorders, as immunocompromised hosts, had higher frequency of infection with SARS‐CoV‐2. Second, hematological patients infected by SARS‐CoV‐2 presented with atypical clinical manifestations, which could be related to the fact that most hematological patients are in an immunosuppressive state due to primary diseases and corresponding treatment. Compared with the clinical symptoms of the general population, 10 patients with hematological disorders presented the higher proportion of fatigue, expectoration, and hemoptysis, related to severe anemia, thrombocytopenia, and infection with other respiratory pathogens. Some patients had atypical evolution characteristics of CT imaging, such as pleural effusion, and absence of typical COVID‐19 CT imaging features, including GOO, crazy‐paving pattern, or consolidation. 11 Notably, increased inflammatory factors is not a specific marker of the deterioration of COVID‐19. Infection with other pathogens and progression of hematological malignancies should also be considered, which led to significantly higher levels of inflammatory factors than the general population. 12 Finally, most infected patients only showed mild symptoms of COVID‐19; however, hematological patients presented with impaired immune response against SARS‐CoV‐2, which was characterized by prolonged viral shedding and weak Ab response, although we did not identify whether anti‐SARS‐CoV‐2 Abs have neutralizing activity. A recent study indicated that the detectable SARS‐CoV‐2 RNA persisted for a median of 20 days in survivors from the general population. 13 Yet, nucleic acid testing remained positive in 4 of 5 survivors in our patients for at least 45 days. A recent retrospective study on the general population reported that an absolute number of T lymphocytes were decreased in nearly all severe cases. 12 Our study revealed that not only CD8+ T cells, but also B cells in all tested patients were below the normal value. Most B lymphocytopenia could be induced by treatment of hematological diseases, such as rituximab, BCMA‐CAR T‐cell therapy, and other immunosuppression therapies, which also suggests that most patients with hematological diseases in active treatment have impaired humoral immunity. Host humoral immunity, especially the production of neutralizing Ab, exerts its protective role by restricting spread/replication of the virus and eventually eliminating the virus at a later phase and preventing reinfection in the future. Concomitant patients in this study were characterized by defective viral clearance and lower level of SARS‐CoV‐2‐specific Abs. These unique features of these patients raise concerns on further clinical management.
The reported fatality rate in the general COVID‐19 population is relatively low, ranging from 1% to 6%. 13 Very high mortality was observed in our case series. Of note, most patients died of the progression of primary diseases or life‐threatening secondary infections, meaning that forced delays or interruption of routine treatment increased the risk of mortality. Consideration of risk and benefit for active intervention in the hematological population during an infectious disease pandemic must be individualized and balanced. Tyrosine kinase inhibitor was well tolerated and effective in our 2 Ph+ ALL patients with COVID‐19. Thus, TKI might represent a good therapeutic possibility in concomitant patients for its anti‐inflammatory and anti‐leukemia activity. However, our study is limited by the small case series, and the safety of TKI used on COVID‐19 patients with Ph+ ALL needs to be further investigated. Given the importance of humoral immunity, the targeted agents that might impair host humoral immunity, such as anti‐CD20 Abs, should be avoided during the treatment. 14 In addition, 2 of 9 concomitant patients died of severe concurrent bacterial infection, indicating the risk of fatality from secondary infection for hematological patients. Whenever possible, screening for concurrent infection with other pathogens is critical.
It is still unclear how long SARS‐CoV‐2 persists during convalescence among immunocompromised hosts. Whether concomitant patients will turn on a chronic clinical course with contiguous potential is particularly challenging and remains uncertain. Further follow‐up and investigation at a larger scale are urgently needed.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81670150 and 81570197), Emergency Research Project of Tongji hospital of Huazhong University of Science and Technology (2020kfyXGYJ045 to JZ), Emergency Research Project of Hubei province (2020FCA006 to Dr WW). The authors would like to thank all members of the study team for their clinical support.
Zhou X, Wang G, Chen L, et al. Clinical characteristics of hematological patients concomitant with COVID‐19. Cancer Sci. 2020;111:3379–3385. 10.1111/cas.14544
Xiaoxi Zhou and Gaoxiang Wang contributed equally to this study.
Contributor Information
Yang Cao, Email: yangcao@tjh.tjmu.edu.cn.
Jianfeng Zhou, Email: jfzhou@tjh.tjmu.edu.cn.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) outbreak. https://www.who.int. Accessed January 30, 2020.
- 2. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahase E. Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. RMD Open. 2020;368:m1036. [DOI] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, J Am Med Assoc. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen T, Wu DI, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin XH, Zheng KI, Pan KH, Xie YP, Zheng MH. COVID‐19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7(4):e351‐e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guidance for Corona Virus Disease 2019: Prevention, Control, Diagnosis and management. http://covid19.21wecan.com/covid19en/c100021/common_list.shtml. Accessed April 7, 2020.
- 9. The protocol of Real‐time reverse‐transcriptase polymerase‐chain‐reaction (RT‐PCR) assay for SARS‐CoV‐2. http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html. Accessed January 21, 2020.
- 10. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: summary of a report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. J Am Med Association. 2020;;323:1239. [DOI] [PubMed] [Google Scholar]
- 11. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper L, Good‐Jacobson KL. Dysregulation of humoral immunity in chronic infection. Immunol Cell Biol. 2020;98(6):456–466. [DOI] [PubMed] [Google Scholar]


