Case series
Coronavirus disease 2019 (COVID‐19) severity appears to parallel the host immune response, with a subset of patients developing COVID‐19 cytokine storm syndrome (CSS). 1 Serum inflammatory cytokines are elevated in COVID‐19 2 , 3 , 4 , 5 and interleukin 6 (IL‐6) appears to play a central role in COVID‐19‐related CSS. 6 , 7 , 8 Based on the success of IL‐6‐receptor blockade for chimeric antigen receptor T‐cell therapy associated cytokine release syndrome (CAR‐T cell CRS), similar strategies using tocilizumab are being investigated in COVID‐19. However, early reports described only modest elevations of IL‐6 of approximately 50 pg/ml (reference range <7 pg/ml) in severe COVID‐19 2 , 3 , 4 , 9 compared to IL‐6 levels often >10 000 pg/l in CAR‐T cell CRS, 10 leading authors to conclude that COVID‐19 pathophysiology is attributable to alternate mechanisms apart from CSS. 11
Two central mechanistic considerations may help resolve this controversy. First, determining if COVID‐19 is associated with markedly elevated IL‐6, in the range seen in CAR‐T cell CRS, is crucial. Second, current trials are focussing on mortality and ventilation endpoints, but data pertaining to the effect of IL‐receptor blockade on inflammatory cytokine levels and cardiorespiratory outcomes are needed to established biological efficacy. We therefore conducted a preliminary evaluation of tocilizumab on inflammatory cytokines including IL‐1β, IL‐6, IL‐10 and tumour necrosis factor alpha (TNF‐α), and physiological parameters in five consecutive patients with severe COVID‐19 CSS. Study approval was obtained from the institutional research ethics board.
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection was confirmed by real‐time reverse transcription polymerase chain reaction from a tracheal aspirate. All patients underwent invasive mechanical ventilation and were diagnosed with acute respiratory distress syndrome (ARDS). 12 Two patients required veno–venous extracorporeal membrane oxygenation (VV‐ECMO) for refractory hypoxaemia. Tocilizumab was administered (single 400 mg dose) 13 as part of clinical care, based on objective manifestations of COVID‐19 CSS: (i) COVID‐19 pneumonia requiring mechanical ventilation; (ii) fever (T max >38°C); (iii) C‐reactive protein (CRP) >100 mg/l; and (iv) peak ferritin >1000 μg/l.
Serum was analysed for IL‐1β, IL‐6, IL‐10 and TNF‐α using the Quanterix® Single Molecule Array (Simoa®) HD‐1 analytical platform daily between 08:00 and 09:00 hours. 14 Routine clinical laboratory data including full blood count, D‐dimer (μg/l), CRP (mg/l), ferritin (μg/l), CD4/CD8 ratio, CD4 (%) and CD8 (%) were collected. Changes in IL‐1β, IL‐6, IL‐10, TNF‐α, and CRP were assessed using the Friedman's test, with a Wilcoxon signed‐ranks test for post hoc comparisons (α = 0·05).
Four patients were male and one was female. The median (range) age was 61 (32–73) years and body mass was 70 (67–75) kg. Four patients presented to hospital with symptoms of fever, all five with cough, and two with headache. All five of the patients had lymphopenia [median (range) 0·6 (0·3–0·8)× 109 cells] and elevated D‐dimer [median (range) 2640 (630–7000) μg/l], ferritin [median (range) 2932 (1051–5638) μg/l] and CRP [median (range) 225 (126–374) mg/l]. The admission median (range) CD4 and CD8 percentages were 42 (35–53)% and 25 (16–33)% respectively, while the CD4/CD8 ratio was 1·46 (1·15–2·69).
All patients had markedly increased peak serum IL‐6 levels during the course of observation [median (range) 2023 (360–13140) pg/ml] (Fig 1), commensurate in magnitude to that observed with CAR‐T cell CRS. 10 Tocilizumab was administered at a median (range) of 2 (1–7) days following mechanical ventilation and was associated with significant reductions in IL‐6 (P = 0·027), IL‐10 (P = 0·009), TNF‐α (P = 0·012), as well as CRP (P < 0·001) (Fig 1). The lymphocyte count increased following tocilizumab administration, but was not statistically significant (+1·0 × 109 cells, 95% confidence interval −0·3 to 1·3; P = 0·063; Fig 1). Statistical analyses of pre‐ and post‐tocilizumab administration D‐dimer and ferritin were not conducted due to missing daily values. The ratio of arterial oxygen tension to fraction of inspired oxygen percentage (PaO2/FiO2), an index of pulmonary gas exchange efficiency, increased from a pre‐treatment value of 123 (range 60–140) to 251 (range 147–310; P = 0·043) on day 6 following tocilizumab administration (Fig 2). Further, mean arterial pressure improved following tocilizumab on days 3 (P = 0·042), 4 (P = 0·042) and 6 (P = 0·041) (Fig 2), which was reflected in markedly decreased intravenous noradrenaline dose requirements. As of 2 June 2020, four of the patients have been discharged home and one died in the intensive care unit.
Fig 1.
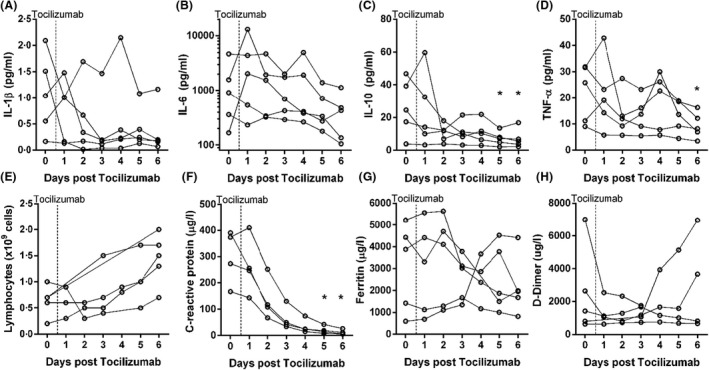
Tocilizumab reduces the concentration of circulating serum inflammatory cytokines and clinical laboratory variables. Top panel (A–D): levels of serum interleukin (IL)‐1β, IL‐6, IL‐10 and tumour necrosis factor alpha (TNF‐α) for each patient. Vertical dashed lines represent the timing of tocilizumab administration relative to daily measurements of cytokines. Bottom Panel (E–H): levels for lymphocyte count, C‐reactive protein, ferritin and D‐dimer for each patient. Vertical dashed lines represent the timing of tocilizumab administration relative to daily measurements of clinical laboratory value. *denotes a significant difference from Day 0, P < 0·05.
Fig 2.
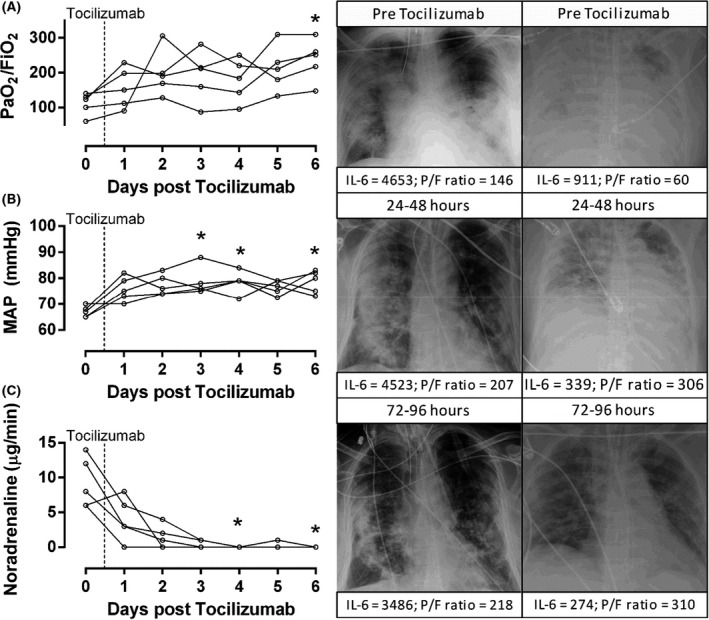
Changes in cardiorespiratory function following tocilizumab. The PaO2/FiO2 ratio, mean arterial pressure (MAP), and noradrenaline dose prior to and following tocilizumab administration. For panels A–C, the physiological variable is displayed on the y‐axis with corresponding units, while days after tocilizumab administration are on the x‐axis. Chest X‐rays are presented for two patients within 24‐h prior to drug administration and then 24–48 h and 72–96 h following drug administration. Individual IL‐6 and PaO2/FiO2 (P/F) ratio data are displayed. For the patient on the left, note that tocilizumab treatment lead to radiographic improvement, despite persistently high inflammation (IL‐6 >3400 pg/ml for all time points). This patient has been discharged home. The patient on the right required VV‐ECMO for refractory hypoxaemia. Tocilizumab led to radiographic improvement in this patient, who has been discharged home. PaO2, partial pressure of arterial oxygen; FIO2, fraction of inspired oxygen. *denotes a significant difference from Day 0, P < 0·05.
Our case series provides evidence that patients with severe COVID‐19 may exhibit hypercytokinaemia, in keeping with ranges described in CAR‐T CRS. We further demonstrate reductions in serum inflammatory markers following tocilizumab administration that correlate with improved clinical parameters. Overall, these preliminary findings provide context for the biological plausibility of IL‐6‐receptor blockade in COVID‐19 CSS. While tocilizumab has been used in IL‐6‐mediated inflammatory conditions, such as the CAR‐T CRS, 10 investigation of the potential efficacy of tocilizumab for treating COVID‐19 to date has been limited to cohorts that are not critically ill (e.g. requiring mechanical ventilation 13 ). To the best of our knowledge, our present case series is the first to demonstrate an association between tocilizumab administration and reductions in multiple key inflammatory cytokines and laboratory variables in the severe spectrum of critically ill patients with COVID‐19 (requiring invasive mechanical ventilation and VV‐ECMO). Importantly, all patients deemed to have COVID‐19 CSS had markedly elevated IL‐6 levels, as high as 13 140 pg/ml, which to our knowledge is one of the highest serum IL‐6 values reported in a patient with COVID‐19 (Fig 1). 15 A recent case study on a single patient with COVID‐19 with ARDS recorded extreme elevations of IL‐6 in peripheral blood (c. 34 000 pg/ml) and pleural fluid (c. 24 000 pg/ml), 15 whereas the majority of studies report median IL‐6 values of approximately 50 pg/ml in cohorts of patients with severe COVID‐19. 9 In fact, Chen et al., 3 reported a median (interquartile range) IL‐6 value of 41.5 (24·8–114.2) pg/ml in an observational study of ‘severe’ COVID‐19, the median of which is >20‐fold lower than that of our present patients.
The results of our present case series should not be used to unequivocally advocate for the use of tocilizumab in COVID‐19, but instead provide context to upcoming and emerging trials of IL‐6‐receptor blockade. Attention should be given to defining COVID‐19 CSS and elucidating the phenotype of patients most likely to benefit from IL‐6‐receptor blockade.
Funding
Dr Sekhon is supported by the Vancouver Coastal Health Research Institute Clinician Scientist Award. This work was funded in part by the Vancouver General Hospital Foundation.
Author contributions
Designed Research: Ryan L. Hoiland, Sophie Stukas, Jennifer Cooper, Cheryl L. Wellington, Mypinder S. Sekhon; Performed Research: Ryan L. Hoiland, Sophie Stukas, Jennifer Cooper, Sonny Thiara, Luke Y. C. Chen, Catherine M. Biggs, Kevin Hay, Agnes Y. Y. Lee, Kamran Shojania, Alym Abdulla, Cheryl L. Wellington, Mypinder S. Sekhon; Wrote the paper: Ryan L. Hoiland, Sophie Stukas, Jennifer Cooper, Luke Y. C. Chen, Cheryl L. Wellington, Mypinder S. Sekhon; Revision for critical intellectual content: Sonny Thiara, Catherine M. Biggs, Kevin Hay, Agnes Y. Y. Lee, Kamran Shojania, Alym Abdulla; Approval of final version: Ryan L. Hoiland, Sophie Stukas, Jennifer Cooper, Sonny Thiara, Luke Y. C. Chen, Catherine M. Biggs, Kevin Hay, Agnes Y. Y. Lee, Kamran Shojania, Alym Abdulla, Cheryl L. Wellington, Mypinder S. Sekhon.
Conflicts of interest
The authors declare no conflicts financial or otherwise.
References
- 1. England JT, Abdulla A, Biggs CM, Lee AY, Hay KA, Hoiland RL, et al. Weathering the COVID‐19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020;15:100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. [Epub ahead of print]. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [Epub ahead of print]. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID‐19) outbreak in china summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 7. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aziz M, Fatima R, Assaly R. Elevated Interleukin‐6 and Severe COVID‐19: a meta‐analysis. J Med Virol. 2020. [Epub ahead of print]. 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leisman DE, Deutschman CS, Legrand M. Facing COVID‐19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46:1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. J Am Med Assoc. 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 13. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD‐1 analyzer: a novel fully automated digital immunoassay analyzer with single‐molecule sensitivity and multiplexing. J Lab Autom. 2016;21:533–47. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Kang K, Gao Y, Ye M, Lan X, Li X, et al. Cytokine levels in the body fluids of a patient with COVID‐19 and acute respiratory distress syndrome: a case report. Ann Intern Med. 2020. [Epub ahead of print]. 10.7326/L20-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]


