Key Points
Question
Does maternal docosahexaenoic acid (DHA) supplementation during the neonatal period improve bronchopulmonary dysplasia–free survival in breastfed infants born before 29 weeks of gestation?
Findings
In this randomized clinical trial that included 461 mothers and 528 preterm infants and was terminated early, maternal intake of DHA during the neonatal period did not significantly improve infants’ bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual age (54.9% vs 61.6% with placebo).
Meaning
Supplementation with DHA in lactating mothers did not significantly improve bronchopulmonary dysplasia–free survival in preterm infants, although study interpretation is limited by early trial termination.
Abstract
Importance
Maternal docosahexaenoic acid (DHA) supplementation may prevent bronchopulmonary dysplasia, but evidence remains inconclusive.
Objective
To determine whether maternal DHA supplementation during the neonatal period improves bronchopulmonary dysplasia–free survival in breastfed infants born before 29 weeks of gestation.
Design, Setting, and Participants
Superiority, placebo-controlled randomized clinical trial at 16 Canadian neonatal intensive care units (June 2015-April 2018 with last infant follow-up in July 2018). Lactating women who delivered before 29 weeks of gestation were enrolled within 72 hours of delivery. The trial intended to enroll 800 mothers, but was stopped earlier.
Interventions
There were 232 mothers (273 infants) assigned to oral capsules providing 1.2 g/d of DHA from randomization to 36 weeks’ postmenstrual age and 229 mothers (255 infants) assigned to placebo capsules.
Main Outcomes and Measures
The primary outcome was bronchopulmonary dysplasia–free survival in infants at 36 weeks’ postmenstrual age. There were 22 secondary outcomes, including mortality and bronchopulmonary dysplasia.
Results
Enrollment was stopped early due to concern for harm based on interim data from this trial and from another trial that was published during the course of this study. Among 461 mothers and their 528 infants (mean gestational age, 26.6 weeks [SD, 1.6 weeks]; 253 [47.9%] females), 375 mothers (81.3%) and 523 infants (99.1%) completed the trial. Overall, 147 of 268 infants (54.9%) in the DHA group vs 157 of 255 infants (61.6%) in the placebo group survived without bronchopulmonary dysplasia (absolute difference, –5.0% [95% CI, –11.6% to 2.6%]; relative risk, 0.91 [95% CI, 0.80 to 1.04], P = .18). Mortality occurred in 6.0% of infants in the DHA group vs 10.2% of infants in the placebo group (absolute difference, –3.9% [95% CI, –6.8% to 1.4%]; relative risk, 0.61 [95% CI, 0.33 to 1.13], P = .12). Bronchopulmonary dysplasia occurred in 41.7% of surviving infants in the DHA group vs 31.4% in the placebo group (absolute difference, 11.5% [95% CI, 2.3% to 23.2%]; relative risk, 1.36 [95% CI, 1.07 to 1.73], P = .01). Of 22 prespecified secondary outcomes, 19 were not significantly different.
Conclusions and Relevance
Among breastfed preterm infants born before 29 weeks of gestation, maternal docosahexaenoic acid supplementation during the neonatal period did not significantly improve bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual age compared with placebo. Study interpretation is limited by early trial termination.
Trial Registration
ClinicalTrials.gov Identifier: NCT02371460
This randomized clinical trial compares the effects of maternal docosahexaenoic acid supplementation from childbirth through 36 weeks’ postmenstrual age vs placebo on bronchopulmonary dysplasia–free survival in breastfed infants born before 29 weeks of gestation.
Introduction
Bronchopulmonary dysplasia is a chronic lung disease that affected approximately half of infants born before 29 weeks of gestation in North America during the past 2 decades.1 The disease increases early childhood mortality, and impairs long-term pulmonary and neurodevelopmental outcomes.2 Optimal nutrition is fundamental to prevent bronchopulmonary dysplasia.3 The long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA) attenuates inflammation, oxidative stress, and improves hyperoxia-induced lung injury and respiratory function in animal studies.4 At birth, premature infants are deficient in DHA due to fetal accretion occurring late during pregnancy.5,6 Low DHA levels in these infants are associated with increased bronchopulmonary dysplasia.7,8 Breastfeeding is an efficient approach to increase DHA stores in preterm infants.9,10 A post hoc analysis of the DHA for the Improvement of Neurodevelopmental Outcome (DINO) trial conducted from 2001 to 2005 suggested that maternal DHA supplementation reduced supplemental oxygen needs at 36 weeks’ postmenstrual age in infants weighting less than 1250 g at birth.11,12 In contrast, the N-3 Fatty Acids for Improvement in Respiratory Outcomes (N3RO) trial conducted from 2012 to 2015 demonstrated that direct enteral DHA administration at birth in infants born before 29 weeks of gestation did not reduce and may even increase the risk of bronchopulmonary dysplasia.13
In light of low dietary DHA intake among women during pregnancy and lactation, the frequent use of over-the-counter DHA, the strong biological plausibility, and the inconsistent results among trials, new evidence is needed to guide recommendations regarding the maternal use of DHA supplements.14,15 The Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants (MOBYDIck) trial aimed to determine whether maternal DHA supplementation during the neonatal period improves bronchopulmonary dysplasia–free survival in breastfed infants born before 29 weeks of gestation.
Methods
Trial Design
This study was a superiority, multicenter randomized placebo-controlled clinical trial involving 16 neonatal intensive care units in Canada. The trial was monitored by a data and safety monitoring board (DSMB) according to charter principles. The DSMB performed 2 safety reviews before the formal interim analysis at the midpoint of recruitment. The trial protocol (appears in Supplement 1) was approved by Health Canada and the research ethics boards of the CHU de Québec-Université Laval (trial coordination center) and all other trial sites. All participating women gave written informed consent.
Participants
Mothers who delivered prematurely between 23 0/7 and 28 6/7 weeks of gestation were screened for eligibility, provided consent, and were enrolled by site investigators or delegates upon admission of infants to the neonatal intensive care unit. Mothers were eligible if they were aged 16 years or older, did not present contraindications for breastfeeding, intended to provide their own breast milk to their infant, and were within 72 hours of delivery at the time of randomization. Mothers were excluded if they took more than 250 mg/d of DHA during the 3 months before enrollment, or if their infant presented with a major congenital or chromosomal abnormality (a complete list of inclusion and exclusion criteria appears in eAppendix 1 in Supplement 2). Because of its strong association with bronchopulmonary dysplasia,16 data on the mother’s race/ethnicity were collected. Race/ethnicity data were self-reported using prespecified categories on the baseline questionnaire.
Treatment Allocation and Masking
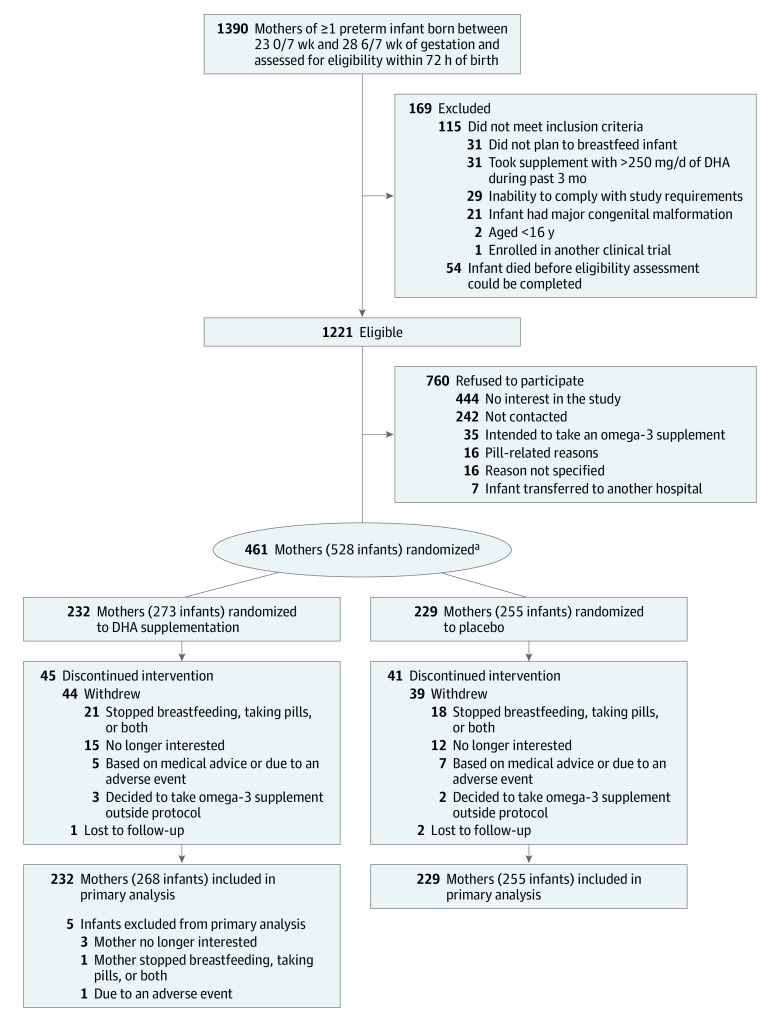
Mothers were randomized in a 1:1 ratio to DHA or placebo according to computer-generated random lists of treatment allocations using variable permuted block sizes of 4 and 6, with a separate list for each center (Figure). Infants from multiple births were assigned to the same group because randomization was performed at the mother level. Randomization lists were generated by an independent statistician and securely kept at the Unité de Recherche Clinique Appliquée, CHU Sainte-Justine. The main trial pharmacist (located at CHU de Québec, Université Laval) coded the medication in advance according to randomization lists for each trial site.
Figure. MOBYDIck Trial Flow Diagram.
MOBYDIck indicates Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants.
aMothers were randomized in a 1:1 ratio to docosahexaenoic acid (DHA) or placebo according to computer-generated random lists of treatment allocations using variable permuted block sizes of 4 and 6 and a separate list for each center.
Site pharmacists were given a blinded randomization list of identification numbers unique to each infant. Treatment bottles were identical and dispensed by site pharmacists blinded to the treatment allocation and were sealed and labeled with the participant’s name, protocol number, study ID, and kit number to allow traceability. Interim analyses and DSMB reports were prepared by biostatisticians not directly involved with the trial. All investigators, research staff, clinicians, pharmacists, the main trial statistician, and participants at each trial site and the data coordinating center were blinded from treatment allocation during the entire trial and data analysis.
Intervention
The mothers received oral capsules providing 1.2 g/d of DHA or placebo capsules (a mix of corn and soy oils) within 72 hours of delivery until their infant reached 36 weeks’ postmenstrual age. Maternal supplementation varied from 8 weeks (eg, for infants born at 28 weeks of gestation) to 13 weeks (eg, for infants born at 23 weeks of gestation). The long-chain polyunsaturated fatty acid–rich oil in the DHA capsules was derived from the algae Schizochytrium sp and had a composition that included approximately 40% DHA (400 mg) and 2.5% eicosapentaenoic acid. The placebo capsules contained 50% soy oil and 50% corn oil (composition appears in eAppendix 2 in Supplement 2). The DHA and placebo capsules tasted and looked identical.
Study Outcomes
The primary outcome was bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual age based on a standardized supplemental oxygen reduction test17 (details appear in eMethods 1-2 in Supplement 2). Bronchopulmonary dysplasia and its severity at 36 weeks’ postmenstrual age were adjudicated by an independent committee. Bronchopulmonary dysplasia severity was classified according to standard criteria.18
The prespecified secondary outcomes up to 40 weeks’ postmenstrual age (see definitions of outcomes in eMethods 1-3 in Supplement 2) were death up to 36 weeks’ postmenstrual age due to any causes; bronchopulmonary dysplasia, bronchopulmonary dysplasia severity, or the need for supplemental oxygen at 36 weeks’ postmenstrual age; days requiring supplemental oxygen or positive pressure respiratory support up to 36 weeks’ postmenstrual age; anthropometric data at 36 weeks’ postmenstrual age; any grade and grade 3 or 4 intraventricular hemorrhage19; clinically or culture-proven sepsis; necrotizing enterocolitis of stage 2 or greater20; significant cholestasis; retinopathy of prematurity at any stage and requiring treatment; periventricular leukomalacia; patent ductus arteriosus requiring surgical ligation; and maternal bleeding requiring treatment.
Data on the amount of days requiring endotracheal ventilation were only collected in a subset of infants, so these data are not included in this article. The prespecified secondary outcomes of days at the hospital from birth to discharge home and data on neurodevelopment, respiratory function, anthropometric measurements; presence of cerebral palsy, blindness, or hearing loss; and number of hospital readmissions and survival at the corrected ages of 18 to 22 months are not reported in this article.
Adverse Events
Adverse events and serious adverse events were reported from randomization to 40 weeks’ postmenstrual age, regardless of severity or relationship to study treatment. Neonatal adverse events were documented in the neonatal case report form and reviewed for safety by the DSMB. Grade 4 intraventricular hemorrhages were obligatorily reported to Health Canada.
Adherence and Assessment of Cointerventions
Maternal adherence to the intervention was monitored by counting capsules returned by mothers on postnatal days 14 and 35 and at 36 weeks’ postmenstrual age. Levels of DHA and fatty acids were measured in breast milk samples collected on postnatal day 14. Maternal omega-3 fatty acid intake from marine foods was assessed using a validated questionnaire.21 Maternal breast milk was provided by tube feeding until the infant was able to feed orally. Enteral feedings were initiated and progressed according to local standards of care, usually within 24 to 48 hours after birth and increased as tolerated. Donor milk or standard formula was used when maternal breast milk could not be provided. The use of parenteral nutrition, including DHA-rich intravenous lipids, and all cointerventions followed local standards of care at each trial site.
Sample Size
In the control group, the proportion of bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was estimated at 45% and mortality was estimated at 10%.22 A sample size of 400 mothers per group was required to detect an absolute difference of 10% in bronchopulmonary dysplasia–free survival in infants, with 80% power and a 2-sided α level of 5%. This 10% difference was based on a pilot study,10 post hoc findings from the DINO trial,12 and a prestudy survey asking Canadian neonatologists how much reduction in the risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age they would consider clinically relevant. Accounting for an inflation factor due to multiple births and intracluster correlations (eMethods 4 in Supplement 2), the trial expected to enroll 450 infants per group.
Statistical Analysis
The unit of analysis was the infant. All randomized mothers along with their infants were included in the analysis, regardless of eligibility and according to their randomization group. Because few missing data were expected, the population data set for the primary outcome corresponds to the randomized population and was restricted to infants assessed for the primary outcome. No imputation was performed for missing data. The population data set for the secondary outcomes is described in eMethods 1-4 in Supplement 2.
The proportions of bronchopulmonary dysplasia–free survival were compared between the DHA and placebo groups (with adjustment for site) using the log binomial regression model with generalized estimating equations (GEEs) to account for the clustering of infant variables. The same model was used to analyze bronchopulmonary dysplasia and its severity in infants who survived at 36 weeks’ postmenstrual age. For most of the other secondary outcomes, the models were not adjusted for site due to convergence issues. However, the results were quite similar for the models that could be fitted (both with and without adjusting for site), suggesting that this adjustment could be ignored.
Subgroup analyses were prespecified for sex and gestational age. An imbalance in mode of delivery between the groups and a qualitative mode of delivery × treatment group interaction were observed by the DSMB during the formal efficacy interim review. Therefore, post hoc stratified analyses were conducted as follows. We used the logistic regression model with GEEs due to convergence issues that precluded fitting either log-binomial regression or log Poisson GEE models. For the subgroup analysis on the primary outcome, we first fitted a logistic regression model with a treatment main effect, a mode of delivery main effect, a treatment × mode of delivery interaction term, and site indicator variables (with GEEs to account for multiple births). The interaction term was significant.
We also fitted models with the prespecified subgroup variables of sex and gestational age. Treatment interaction was significant for each of these variables. The final model included treatment main effect, mode of delivery main effect, gestational age main effect, all double interaction terms, and site indicator variables. Sex and the interaction among treatment, mode of delivery, and gestational age were dropped from this model because they no longer significantly improved the model fit. The final model and the prespecified models for the subgroups appear in Supplement 2. When logistic regression was used, odds ratios were estimated instead of relative risks (RRs). The same model was used for stratified analyses on the secondary outcomes. Because of the potential for type I error due to multiple comparisons, the findings for the secondary outcomes should be interpreted as exploratory.
For all analyses, a 2-sided P ≤ .05 was considered significant. The analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Interim Analysis and Early Trial Termination
During this trial, the results from the N3RO trial became available, suggesting a higher risk of bronchopulmonary dysplasia from DHA supplementation.13 After the release of the N3RO trial results raising a concern for harm, the DSMB requested descriptive statistics on the primary outcome by groups. After this review, a post hoc conditional power analysis was conducted using different scenarios of sample sizes and alternative hypotheses. Based on these analyses, the DSMB recommended continuing the trial as planned.
An efficacy interim analysis was prespecified for the primary outcome when approximately 50% of the trial participants (or 400 mothers) had been enrolled in the trial. At the interim analysis, the proportion of infants who survived free of bronchopulmonary dysplasia was 57.8% (119/206 infants) in the DHA group vs 62.4% (121/194 infants) in the placebo group. Although the P value of .28 for this difference was above the threshold for stopping defined by O’Brien-Fleming (P < .01), the emerging data from this trial were consistent with the results from the N3RO trial.13
A post hoc conditional power analysis was conducted (using East version 6.3; Cytel) at the request of the DSMB. Conditional power was less than 0.01% and 6% under 2 alternative hypotheses of a true absolute difference between groups of 5% and 10%, respectively, using the observed proportion in the placebo group. Therefore, recruitment of new participants was prematurely terminated by the DSMB on April 4, 2018, due to concern of harm for future participants based on interim data favoring the placebo group, findings from the N3RO trial,13 and the very low probability of benefit from DHA supplementation. The final conditional power analyses were repeated using the same methods specified above.
Results
Characteristics of Participants
A total of 461 mothers were enrolled from June 23, 2015, through April 3, 2018, with last infant follow-up in July 2018. There were 232 mothers (and their 273 infants) assigned to the DHA group and 229 mothers (and their 255 infants) assigned to the placebo group. Seven randomized mothers retrospectively did not meet eligibility criteria but were kept in the analysis: 5 were not enrolled within 72 hours of delivery and 2 took more than 250 mg of DHA supplements during the 3 months prior to enrollment. Five infants from 4 mothers in the DHA group were withdrawn based on parental request before the primary outcome assessment (Figure). Of 528 infants, these 5 (0.9%) were excluded from the primary outcome analysis. The majority of maternal and infant baseline characteristics were similar between groups. However, there was a higher percentage of cesarean deliveries in the DHA group (69.8%) compared with the control group (55.5%) (Table 1 and eTables 1-2 in Supplement 2). There were 375 mothers (81.3%) and 523 infants (99.1%) who completed the trial.
Table 1. Baseline Characteristics of the Mothers and Their Infants.
| Characteristic | No. (%)a | |
|---|---|---|
| Docosahexaenoic acid | Placebo | |
| Mothers | (n = 232) | (n = 229) |
| Age, mean (SD), y | 30.8 (5.3) | 31.1 (5.3) |
| Race/ethnicityb,c | (n = 230) | (n = 226) |
| White | 131 (56.5) | 139 (60.7) |
| Native African or African American | 25 (10.8) | 26 (11.4) |
| Asian | 34 (14.7) | 23 (10.0) |
| Arab or West Asian | 10 (4.3) | 6 (2.6) |
| Latin American | 6 (2.6) | 7 (3.1) |
| Native or aboriginal | 13 (5.6) | 14 (6.1) |
| Mixed ethnicity | 11 (4.7) | 11 (4.8) |
| Antenatal corticosteroidsc | (n = 230) 216 (93.1) |
(n = 226) 211 (92.1) |
| Timing of antenatal corticosteroids prior to deliveryc | (n = 229) | (n = 225) |
| <24 h | 75 (32.3) | 81 (35.4) |
| ≥24 h and <7 d | 91 (39.2) | 82 (35.8) |
| ≥7 d | 49 (21.1) | 47 (20.5) |
| Spontaneous rupture of membranesc | (n = 224) 117 (50.4) |
(n = 224) 127 (55.5) |
| Clinical suspicion of chorioamnionitisc | (n = 224) 33 (14.2) |
(n = 218) 23 (10.0) |
| Temperature during labor >38 °Cc | (n = 230) 12 (5.2) |
(n = 226) 9 (3.9) |
| Presence of laborc | (n = 230) 153 (65.9) |
(n = 226) 160 (69.9) |
| Cesarean deliveryc | (n = 230) 162 (69.8) |
(n = 226) 127 (55.5) |
| Multiple births | ||
| Singleton | 194 (83.6) | 200 (87.3) |
| Twins | 31 (13.4) | 28 (12.2) |
| Triplets | 6 (2.6) | 1 (0.4) |
| Quadruplets | 1 (0.4) | 0 |
| Infants | (n = 273) | (n = 255) |
| Gestational age | ||
| Mean (SD), wk | 26.7 (1.5) | 26.4 (1.6) |
| <27 wk | 142 (52.0) | 147 (57.6) |
| Sex | ||
| Male | 138 (50.5) | 137 (53.7) |
| Female | 135 (49.5) | 118 (46.3) |
| Birth weight | ||
| Mean (SD), g | 898.2 (242.2) | 891.8 (237.4) |
| <10th percentile for gestational age | 27 (9.9) | 21 (8.2) |
| Need for intubation in delivery room | 98 (35.9) | 107 (42.0) |
| Apgar score <7 at 5 min after birthc,d | (n = 272) 116 (42.5) |
(n = 252) 119 (46.7) |
Unless otherwise indicated.
Self-reported by participants using multiple choice categories provided on the baseline questionnaire.
There were missing data but the percentages were calculated using the entire population (by each group) as the denominator.
Reflects the skin color or appearance, heart rate, response to stimulation, muscle tone, and respiratory effort. A score of less than 7 is considered abnormal.
The mean maternal adherence to the intervention was 73.1% (SD, 28.2%) of expected doses overall (Table 2 and eTable 3 in Supplement 2). The DHA mean percentage of total fatty acids in the expressed breast milk samples was 0.95% (95% CI, 0.89%-1.01%) in the DHA group on postnatal day 14 and 0.34% (95% CI, 0.31% to 0.37%) in the placebo group (mean difference, 0.61% [95% CI, 0.54% to 0.68%], P < .001). More than 98% of infants received maternal breast milk before 2 days of age. The age at discontinuation of parenteral nutrition, volume of maternal breast milk received by infants at 2 and 6 weeks postdelivery, and the proportion of infants who were still receiving maternal breast milk at 36 weeks’ postmenstrual age appear in Table 2. The daily amounts of maternal breast milk received by infants from birth to 15 days of age appear in the eFigure and eTable 4 in Supplement 2.
Table 2. Maternal Adherence to Intervention and Infant Nutritional Data.
| Docosahexaenoic acid | Placebo | |
|---|---|---|
| Mothers | (n = 232) | (n = 229) |
| Ratio of capsules taken up to 36 weeks’ postmenstrual age, mean (SD)a | (n = 210) 72.9 (28.7) |
(n = 201) 73.2 (27.7) |
| Provided a breast milk sample, No. (%) | 198 (85.3) | 193 (84.3) |
| Time after delivery, mean (SD), d | (n = 198) 14.7 (3.7) |
(n = 193) 14.8 (2.9) |
| Fatty acid levels in breast milk, mean (95% CI) | (n = 196) | (n = 193) |
| DHA, mg/mL | 0.33 (0.30-0.35) | 0.12 (0.11-0.13) |
| DHA, % of total fatty acids | 0.95 (0.89-1.01) | 0.34 (0.31-0.37) |
| Total, mg/mL | 35.28 (33.59-36.97) | 36.72 (34.82-38.61) |
| Infants | (n = 273) | (n = 255) |
| Intravenous DHA-rich lipids before 36 weeks’ postmenstrual age, No. (%) | 144 (52.8) | 128 (50.2) |
| Duration, mean (SD), d | (n = 144) 20.2 (13.3) |
(n = 128) 22.4 (14.7) |
| Age at discontinuation of parenteral nutrition, mean (SD), d | 26.3 (19.0) | 25.0 (19.5) |
| Received any enteral feeding, No. (%)b | (n = 273) 272 (99.6) |
(n = 254) 252 (98.8) |
| Age at first enteral feeding, mean (SD), d | (n = 272) 1.6 (2.0) |
(n = 252) 1.4 (1.6) |
| Received maternal breast milk, No. (%)b | (n = 273) 268 (98.2) |
(n = 254) 250 (98.0) |
| Age at first feeding with maternal breast milk, mean (SD), d | (n = 268) 2.5 (2.4) |
(n = 250) 2.3 (2.2) |
| Maternal breast milk at 36 weeks’ postmenstrual age ±4 d, No. (%)b | (n = 246) | (n = 223) |
| Received any | 182 (66.7) | 153 (60.0) |
| Received exclusively | 129 (47.3) | 113 (44.3) |
| Amount of expressed breast milk provided, median (IQR), mL/kg/dc | ||
| At 2 wk | (n = 264) 60 (19-100) |
(n = 241) 61 (19-100) |
| At 6 wk | (n = 251) 126 (37-148) |
(n = 229) 121 (42-147) |
Abbreviation: IQR, interquartile range.
Based on available pill count from supplement distribution up to 36 weeks’ postmenstrual age or death of the infant.
There were missing data but the percentages were calculated using the entire population (by each group) as the denominator.
Estimated from the mean amount of expressed breast milk received per day during the week/average infant weight for the week and does not take into account direct breastfeeding.
Outcomes
A total of 147 of 268 infants (54.9%) in the DHA group survived without bronchopulmonary dysplasia at 36 weeks’ postmenstrual age compared with 157 of 255 infants (61.6%) in the placebo group (absolute difference, –5.0% [95% CI, –11.6% to 2.6%]; RR, 0.91 [95% CI, 0.80 to 1.04], P = .18; Table 3). Final conditional power was less than 0.01% for both alternative hypotheses.
Table 3. Primary and Major Secondary Outcomes.
| No./total (%) | Absolute difference, % (95% CI) | Relative risk (95% CI) | P value | ||
|---|---|---|---|---|---|
| Docosahexaenoic acid | Placebo | ||||
| Primary outcome | |||||
| Bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual agea | 147/268 (54.9) | 157/255 (61.6) | –5.0 (–11.6 to 2.6)b | 0.91 (0.80 to 1.04)b | .18 |
| Major secondary outcomes | |||||
| Death before 36 weeks’ postmenstrual agea | 16/268 (6.0) | 26/255 (10.2) | –3.9 (–6.8 to 1.4)c | 0.61 (0.33 to 1.13)c | .12 |
| Bronchopulmonary dysplasia at 36 weeks’ postmenstrual aged | |||||
| Physiologicale | 105/252 (41.7) | 72/229 (31.4) | 11.5 (2.3 to 23.2)b | 1.36 (1.07 to 1.73)b | .01 |
| Level of severityf | |||||
| Severeg | 88/252 (34.9) | 58/229 (25.3) | 10.3 (1.7 to 21.7)b | 1.41 (1.07 to 1.86)b | .02 |
| Moderate | 16/252 (6.4) | 13/229 (5.7) | 0.7 (–2.5 to 7.2)c | 1.12 (0.55 to 2.27)c | .75 |
| Mild | 70/252 (27.8) | 78/229 (34.1) | –5.5 (–10.7 to 1.3)b | 0.81 (0.62 to 1.04)b | .10 |
Eight deaths (1 in the docosahexaenoic acid group and 7 in the placebo group) were potentially related to bronchopulmonary dysplasia (eTable 5 in Supplement 2). The denominators excluded infants when the primary outcome was not available at 36 weeks’ postmenstrual age (5 in the docosahexaenoic acid group).
Model included treatment main effect, site indicator, and clustering of infant variables. The absolute difference or relative risk was derived from the model and accounted for site indicator and clustering of infant variables.
Model included treatment main effect and clustering of infant variables. The absolute difference or relative risk was derived from the model and accounted for clustering of infant variables.
Denominators excluded infants when the primary outcome was not available at 36 weeks’ postmenstrual age or because of death.
Defined according to the need for supplemental oxygen or ventilation to maintain an oxygen saturation level of 90% or greater (during the supplemental oxygen reduction test) at 36 weeks’ postmenstrual age.
Classified according to criteria18 from the National Institute of Child Health and Human Development.
Defined as the need for supplemental oxygen for at least 28 days up to 36 weeks’ postmenstrual age plus the need for oxygen (≥30%), positive pressure ventilation, or both at 36 weeks’ postmenstrual age.
Bronchopulmonary dysplasia occurred in 105 of 252 infants (41.7%) who survived to 36 weeks’ postmenstrual age in the DHA group vs 72 of 229 infants (31.4%) in the placebo group (absolute difference, 11.5% [95% CI, 2.3% to 23.2%]; RR, 1.36 [95% CI, 1.07 to 1.73], P = .01; Table 3). Rates of severe bronchopulmonary dysplasia were 34.9% in the DHA group vs 25.3% in the placebo group (absolute difference, 10.3% [95% CI, 1.7% to 21.7%]; RR, 1.41 [95% CI, 1.07 to 1.86], P = .02). Among the 44 deaths before 40 weeks’ postmenstrual age, 42 deaths occurred before 36 weeks’ postmenstrual age (eTable 5 in Supplement 2). Mortality before 36 weeks’ postmenstrual age was 6.0% (n = 16) in the DHA group vs 10.2% (n = 26) in the placebo group (absolute difference, –3.9% [95% CI, –6.8% to 1.4%]; RR, 0.61 [95% CI, 0.33 to 1.13], P = .12).
The majority of effects of DHA supplementation relative to placebo on other secondary outcomes were not significantly different (Table 4). However, the rate of grade 3 or 4 intraventricular hemorrhage was significantly different and occurred in 21 of 273 infants (7.7%) in the DHA group vs 41 of 255 infants (16.1%) in the placebo group (absolute difference, –8.4% [95% CI, –11.4% to –3.2%]; RR, 0.48 [95% CI, 0.29 to 0.80], P = .005).
Table 4. Other Secondary Outcomes.
| No./total (%)a | Absolute difference, % (95% CI)b | Relative risk (95% CI)b | P value | ||
|---|---|---|---|---|---|
| Docosahexaenoic acid | Placebo | ||||
| Infantsc | |||||
| Intraventricular hemorrhaged | |||||
| Any grade | 87/273 (31.9) | 99/255 (38.8) | –3.6 (–9.5 to 4.0)e | 0.89 (0.70 to 1.12)e | .32 |
| Grade 3 or 4 | 21/273 (7.7) | 41/255 (16.1) | –8.4 (–11.4 to –3.2) | 0.48 (0.29 to 0.80) | .005 |
| Clinically or culture-proven sepsisf,g | 104/257 (40.5) | 87/234 (37.2) | 4.1 (–4.3 to 14.7) | 1.11 (0.88 to 1.40) | .37 |
| Necrotizing enterocolitis ≥stage 2g,h | 14/260 (5.4) | 7/231 (3.0) | 3.0 (–0.6 to 12.2) | 2.02 (0.79 to 5.18) | .14 |
| Highest conjugated (direct) bilirubin level ≥2 mg/dLg | 29/250 (11.6) | 31/227 (13.7) | –1.7 (–6.2 to 5.8) | 0.88 (0.54 to 1.42) | .60 |
| Supplemental oxygen needs at 36 weeks’ postmenstrual agei | 90/252 (35.7) | 72/229 (31.4) | 5.3 (–2.4 to 15.1)e | 1.18 (0.92 to 1.50)e | .20 |
| Prior to 36 weeks’ postmenstrual age, mean (SD), di | |||||
| Requiring supplemental oxygen | (n = 256) 46.9 (25.2) |
(n = 229) 48.2 (25.8) |
–0.4 (–5.1 to 4.2)e | .85 | |
| Requiring invasive or noninvasive positive pressure respiratory support | (n = 257) 56.8 (18.9) |
(n = 229) 57.9 (21.4) |
–0.5 (–4.1 to 3.2)e | .80 | |
| Retinopathy of prematurityg | |||||
| Any stage | 128/252 (50.8) | 109/225 (48.4) | 3.0 (–4.5 to 11.9)e | 1.07 (0.90 to 1.26)e | .46 |
| Requiring treatment | 20/250 (8.0) | 19/225 (8.4) | –0.2 (–4.1 to 6.9) | 0.97 (0.52 to 1.81) | .93 |
| Periventricular leukomalaciag | 6/254 (2.4) | 8/228 (3.5) | –1.0 (–2.5 to 3.7) | 0.71 (0.24 to 2.11) | .54 |
| Posthemorrhagic hydrocephalusg | 7/256 (2.7) | 7/231 (3.0) | –0.3 (–2.1 to 4.6) | 0.90 (0.32 to 2.53) | .84 |
| Patent ductus arteriosus requiring surgical ligation | 20/273 (7.3) | 11/255 (4.3) | 3.3 (–0.6 to 11.7) | 1.81 (0.85 to 3.85) | .12 |
| Before 40 weeks’ postmenstrual agej | |||||
| Discharged home without requiring oxygen | 100/265 (37.7) | 101/248 (40.7) | –5.4 (–11.9 to 2.6)e | 0.86 (0.70 to 1.07)e | .17 |
| Discharged home requiring oxygen, respiratory support, or both | 9/263 (3.4) | 8/246 (3.3) | 0.2 (–2.0 to 5.9) | 1.05 (0.40 to 2.77) | .92 |
| Weight at 36 weeks’ postmenstrual age, mean (SD), gi | (n = 246) 2230.0 (410.0) |
(n = 222) 2240.0 (356) |
–18.7 (–89.8 to 52.4)e | .61 | |
| Mothers | |||||
| Bleeding requiring treatment or hospitalizationk | 2/232 (0.9) | 4/229 (1.8) | –0.9 (–1.6 to 2.9)l | 0.49 (0.09 to 2.67)l | .41 |
Unless otherwise indicated.
Model included treatment main effect and clustering of infant variables unless otherwise specified. The absolute difference or relative risk was derived from the model and accounted for clustering of infant variables.
Denominator variability between outcomes is related to the number of infants for whom the outcome occurred before death.
Worst grade from birth based on criteria from Papile et al.19 A diagnosis of grade 3 or 4 was made before randomization in 8 infants.
Model included treatment main effect, site indicator, and clustering of infant variables. The absolute difference or relative risk was derived from the model and accounted for site indicator and clustering of infant variables.
At least 1 episode of sepsis within 72 hours of delivery that required intravenous antibiotic treatment for 5 days or longer.
Denominators excluded infants when outcome of interest was not available at 40 weeks’ postmenstrual age and infants who did not have the condition and died before 40 weeks’ postmenstrual age.
According to criteria from Bell et al.20
Denominators excluded infants when the primary outcome was not available at 36 weeks’ postmenstrual age and infants who died before 36 weeks’ postmenstrual age.
Denominators excluded infants when the outcome of interest was not available at 40 weeks’ postmenstrual age.
Reported as maternal serious adverse events.
Model included treatment main effect.
The prespecified subgroup analyses by sex (eTable 6 in Supplement 2) and gestational age (eTable 7 in Supplement 2) are presented for the primary outcome. Post hoc analyses of the primary outcome, stratified by mode of delivery and gestational age, detected significant interactions between treatment groups and gestational age (<27 weeks or 27-<29 weeks; P = .02 for interaction), and between treatment groups and mode of delivery (ie, vaginal or cesarean delivery; P < .001 for interaction). An opposite effect of DHA supplementation on bronchopulmonary dysplasia–free survival was observed between infants born vaginally vs via cesarean delivery. Among infants born vaginally before 27 weeks of gestation, bronchopulmonary dysplasia–free survival was improved in the DHA group compared with the placebo group (57.5% vs 32.8%, respectively; odds ratio, 3.26 [95% CI, 1.41-7.52]; eTable 8 in Supplement 2). The post hoc stratified analyses for the secondary outcomes appear in eTable 8 and eTable 9 in Supplement 2.
Adverse Events
No neonatal adverse events or serious adverse events were reported to be related to the intervention by investigators. A total of 138 adverse events were reported in 87 of 461 mothers (18.9%). In addition, there were 19 serious adverse events reported in 18 of 461 mothers (3.9%). These events were reported similarly in both groups (eTables 10-11 in Supplement 2). Headache (6.9% in the DHA group vs 6.1% in the placebo group) and gastrointestinal disorders (6.9% vs 5.7%, respectively) were the most frequently reported adverse events.
Discussion
In this randomized clinical trial, the oral intake of 1.2 g/d of DHA by lactating mothers during the neonatal period did not improve bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual age in their infants born before 29 weeks of gestation. These findings do not support the post hoc findings from the DINO trial.12 Rather, the results from the current trial are more consistent with the N3RO trial13 that showed no improvement in bronchopulmonary dysplasia from direct enteral DHA administration. Contrary to the direct pharmacological approach used in the N3RO trial, the current trial used a nutritional approach involving indirect DHA supplementation to preterm infants.
The current trial was stopped early because data from the interim analysis favored the placebo group, and in view of a concern for harm suggested both by this trial and by the N3RO trial. Therefore, the primary hypothesis was disproved before the planned enrollment, demonstrating no benefit from DHA supplementation on bronchopulmonary dysplasia–free survival, regardless of the mode of enteral administration. In addition, clinical benefits from DHA supplementation were not detected in the majority of the secondary outcomes. However, there was a decrease in severe intraventricular hemorrhage in the DHA group. However, the 2 events are unlikely to be causally related, considering that the intraventricular hemorrhage likely preceded the study intervention.23
Lower mortality in the DHA group may have increased the proportion of infants with severe bronchopulmonary dysplasia. However, to the best of our knowledge, there are no data in the literature supporting that DHA supplementation reduces the rate of death in this population. Moreover, deaths in this trial were relatively infrequent, and the difference was not statistically significant. Only a subset of these deaths was potentially related to bronchopulmonary dysplasia. In the N3RO trial, mortality before 36 weeks’ postmenstrual age was not significantly different between the DHA group (n = 631) and the placebo group (n = 642) (6.2% vs 4.5%, respectively; RR, 1.33 [95% CI, 0.88 to 2.12], P = .23).13 Therefore, in the absence of an a priori hypothesis and supporting data, an effect of DHA on survival is hypothetical and requires confirmation.
The post hoc finding of the differential effect of DHA supplementation vs placebo on bronchopulmonary dysplasia–free survival, as well as on the secondary outcome of bronchopulmonary dysplasia in infants born vaginally vs those born via cesarean delivery is likely due to chance. Alternatively, it is biologically plausible that the response to DHA supplementation is modulated by maternal and fetal conditions influencing the mode of delivery, such as chorioamnionitis, intrauterine growth restriction, and preeclampsia, and that are themselves associated with antenatal and postnatal inflammation and oxidative stress in the infant.24,25,26,27 These findings deserve validation because they may need to be taken into account when orienting interventions for bronchopulmonary dysplasia.
Limitations
This study has several limitations. First, the DHA levels in infants were not measured because this was thought to be too invasive. This precludes a better understanding of why DHA did not reduce bronchopulmonary dysplasia–free survival. However, the final results from this trial can still be pragmatically interpreted. Levels of DHA in breast milk from mothers in the placebo group ranged from 0.2% to 0.45%, which is expectedly low and similar to levels reported in women not receiving supplementation on a typical Western diet.28,29,30,31 Thus, it is unlikely that infants in the placebo group were exposed to DHA contamination (either through their mothers’ diet or over-the-counter use of DHA supplements).
Conversely, despite an overall maternal adherence of 73%, breast milk samples collected at 2 weeks in 84% of the mothers reached the targeted 1% DHA of total fatty acids in the DHA group. This increase in breast milk DHA is sufficient to raise DHA levels in infants at steady state comparably with direct supplementation of the infants.9,10,11 Furthermore, the intake of maternal milk by infants in both groups was well within standards of practice in neonatal intensive care units in Canada and also elsewhere. Thus, practically speaking it is unlikely that the study intervention failed.
Second, about half of the infants in this trial received intravenous DHA-rich lipids as per the standard of clinical care, and because the use of these lipids does not differ between the DHA group and the placebo group, it is also unlikely that their frequent use explains the negative findings in this trial.
Conclusions
Among breastfed preterm infants born before 29 weeks of gestation, maternal docosahexaenoic acid supplementation during the neonatal period did not significantly improve bronchopulmonary dysplasia–free survival at 36 weeks’ postmenstrual age compared with placebo. Study interpretation is limited by early trial termination.
Trial protocol and statistical analysis plan
eAppendix 1. Inclusion and exclusion criteria
eAppendix 2. Fatty acid composition of study DHA and placebo capsules
eAppendix 3. MOBYDIck trial collaborators and affiliations
eAppendix 4. Principal investigators and co-investigators
eAppendix 5. Sites, site investigators and coordinators
eAppendix 6. Research ethics boards that approved the study at each site
eAppendix 7. MOBYDIck trial recruitment by sites
eMethods 1. Definitions of primary and major secondary outcomes as reported
eMethods 2. Supplemental oxygen reduction test
eMethods 3. Definitions of neonatal secondary outcomes (listed on Table 4)
eMethods 4. Statistical analysis
eTable 1. Additional baseline characteristics in mothers
eTable 2. Additional baseline characteristics in infants
eTable 3. Additional information for the adherence to intervention
eFigure. Volumes of maternal breast milk received by infants from birth to 15 days of age
eTable 4. Corresponding number of infants in whom maternal breast milk data were available for each day of age
eTable 5. Causes of mortality
eTable 6. Prespecified analysis of primary and major secondary outcomes stratified by sex
eTable 7. Prespecified analysis of primary and major secondary outcomes stratified by gestational age
eTable 8. Post-hoc analysis of primary and major secondary outcomes stratified by gestational age and mode of delivery
eTable 9. Post-hoc analysis of other secondary outcomes stratified by gestational age and mode of delivery
eTable 10. Maternal adverse events
eTable 11. Maternal serious adverse events
eReferences
Data sharing statement
References
- 1.Siffel C, Kistler KD, Lewis JFM, Sarda SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med. Published online August 9, 2019. doi: 10.1080/14767058.2019.1646240 [DOI] [PubMed] [Google Scholar]
- 2.Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet. 2017;389(10079):1649-1659. doi: 10.1016/S0140-6736(17)30312-4 [DOI] [PubMed] [Google Scholar]
- 3.Higgins RD, Jobe AH, Koso-Thomas M, et al. . Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300-308. doi: 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentine CJ. Maternal dietary DHA supplementation to improve inflammatory outcomes in the preterm infant. Adv Nutr. 2012;3(3):370-376. doi: 10.3945/an.111.001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman-van Drongelen MM, van Houwelingen AC, Kester AD, Hasaart TH, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids in preterm infants: status at birth and its influence on postnatal levels. J Pediatr. 1995;126(4):611-618. doi: 10.1016/S0022-3476(95)70363-2 [DOI] [PubMed] [Google Scholar]
- 6.Lapillonne A, Groh-Wargo S, Gonzalez CHL, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr. 2013;162(3)(suppl):S37-S47. doi: 10.1016/j.jpeds.2012.11.052 [DOI] [PubMed] [Google Scholar]
- 7.Martin CR, Dasilva DA, Cluette-Brown JE, et al. . Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159(5):743-749.e1, e2. doi: 10.1016/j.jpeds.2011.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Lavoie PM, Lacaze-Masmonteil T, Rhainds M, Marc I. Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: a systematic review. Pediatrics. 2014;134(1):120-134. doi: 10.1542/peds.2014-0459 [DOI] [PubMed] [Google Scholar]
- 9.Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids. 2015;99:1-6. doi: 10.1016/j.plefa.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Marc I, Plourde M, Lucas M, et al. . Early docosahexaenoic acid supplementation of mothers during lactation leads to high plasma concentrations in very preterm infants. J Nutr. 2011;141(2):231-236. doi: 10.3945/jn.110.125880 [DOI] [PubMed] [Google Scholar]
- 11.Makrides M, Gibson RA, McPhee AJ, et al. . Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301(2):175-182. doi: 10.1001/jama.2008.945 [DOI] [PubMed] [Google Scholar]
- 12.Manley BJ, Makrides M, Collins CT, et al. ; DINO Steering Committee . High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics. 2011;128(1):e71-e77. doi: 10.1542/peds.2010-2405 [DOI] [PubMed] [Google Scholar]
- 13.Collins CT, Makrides M, McPhee AJ, et al. . Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N Engl J Med. 2017;376(13):1245-1255. doi: 10.1056/NEJMoa1611942 [DOI] [PubMed] [Google Scholar]
- 14.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89(2):678S-684S. doi: 10.3945/ajcn.2008.26811E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordgren TM, Lyden E, Anderson-Berry A, Hanson C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: potential for deficiency? Nutrients. 2017;9(3):e197-e209. doi: 10.3390/nu9030197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janevic T, Zeitlin J, Auger N, et al. . Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr. 2018;172(11):1061-1069. doi: 10.1001/jamapediatrics.2018.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23(6):451-456. doi: 10.1038/sj.jp.7210963 [DOI] [PubMed] [Google Scholar]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723-1729. doi: 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 20.Bell MJ, Ternberg JL, Feigin RD, et al. . Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. doi: 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas M, Asselin G, Mérette C, Poulin MJ, Dodin S. Validation of an FFQ for evaluation of EPA and DHA intake. Public Health Nutr. 2009;12(10):1783-1790. doi: 10.1017/S1368980008004333 [DOI] [PubMed] [Google Scholar]
- 22.Canadian Neonatal Network 2014 Annual report. Accessed August 15, 2014. http://www.canadianneonatalnetwork.org/Portal/LinkClick.aspx?fileticket=eGgxmMubxjk%3d&tabid=39
- 23.Benders MJ, Kersbergen KJ, de Vries LS. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin Perinatol. 2014;41(1):69-82. doi: 10.1016/j.clp.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Chang BA, Huang Q, Quan J, et al. . Early inflammation in the absence of overt infection in preterm neonates exposed to intensive care. Cytokine. 2011;56(3):621-626. doi: 10.1016/j.cyto.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leroy S, Caumette E, Waddington C, Hébert A, Brant R, Lavoie PM. A time-based analysis of inflammation in infants at risk of bronchopulmonary dysplasia. J Pediatr. 2018;192:60-65.e1, e61. doi: 10.1016/j.jpeds.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 26.Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154(1):39-43.e3, e33. doi: 10.1016/j.jpeds.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 27.Valentine CJ, Dingess KA, Kleiman J, Morrow AL, Rogers LK. A randomized trial of maternal docosahexaenoic acid supplementation to reduce inflammation in extremely preterm infants. J Pediatr Gastroenterol Nutr. 2019;69(3):388-392. doi: 10.1097/MPG.0000000000002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457-1464. doi: 10.1093/ajcn/85.6.1457 [DOI] [PubMed] [Google Scholar]
- 29.Innis SM, Elias SL. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am J Clin Nutr. 2003;77(2):473-478. doi: 10.1093/ajcn/77.2.473 [DOI] [PubMed] [Google Scholar]
- 30.Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71(1)(suppl):292S-299S. doi: 10.1093/ajcn/71.1.292s [DOI] [PubMed] [Google Scholar]
- 31.Makrides M, Neumann MA, Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr. 1996;50(6):352-357. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eAppendix 1. Inclusion and exclusion criteria
eAppendix 2. Fatty acid composition of study DHA and placebo capsules
eAppendix 3. MOBYDIck trial collaborators and affiliations
eAppendix 4. Principal investigators and co-investigators
eAppendix 5. Sites, site investigators and coordinators
eAppendix 6. Research ethics boards that approved the study at each site
eAppendix 7. MOBYDIck trial recruitment by sites
eMethods 1. Definitions of primary and major secondary outcomes as reported
eMethods 2. Supplemental oxygen reduction test
eMethods 3. Definitions of neonatal secondary outcomes (listed on Table 4)
eMethods 4. Statistical analysis
eTable 1. Additional baseline characteristics in mothers
eTable 2. Additional baseline characteristics in infants
eTable 3. Additional information for the adherence to intervention
eFigure. Volumes of maternal breast milk received by infants from birth to 15 days of age
eTable 4. Corresponding number of infants in whom maternal breast milk data were available for each day of age
eTable 5. Causes of mortality
eTable 6. Prespecified analysis of primary and major secondary outcomes stratified by sex
eTable 7. Prespecified analysis of primary and major secondary outcomes stratified by gestational age
eTable 8. Post-hoc analysis of primary and major secondary outcomes stratified by gestational age and mode of delivery
eTable 9. Post-hoc analysis of other secondary outcomes stratified by gestational age and mode of delivery
eTable 10. Maternal adverse events
eTable 11. Maternal serious adverse events
eReferences
Data sharing statement