Abstract
The transradial approach is recommended as a first choice in coronary catheterizations and interventions, for among other reasons, the reduction in the number of local complications. A head-to-head comparison of the reverse Barbeau test (RBT) and duplex ultrasonography (DUSG) for the detection of post-procedural radial artery patency and occlusion has not yet been evaluated. In 500 patients from our same-day discharge program (age 65 ± 9.4 years, 148 women), radial artery patency and occlusion, compression time, haematomas, and other local complications were evaluated. Radial artery patency was confirmed in 495 patients (99.0%), and complete radial artery occlusion (RAO) was detected in 2 (0.4%) patients using both methods. In 3 patients (0.6%), the RBT was negative, while incomplete RAO was detected by DUSG. Superficial haematomas (˃ 5 but ≤10 cm) were found in 27 (5.4%) patients. There were no other local complications. Detection of radial artery patency and occlusion using the RBT and DUSG was comparable. The incidence of RAO in our study was extremely low. Thanks to its simplicity, the RBT has the potential to be used as the first method of detection of radial occlusion after coronary catheterizations.
Keywords: Radial approach, Radial artery patency, Radial artery occlusion, Reverse Barbeau test, Duplex ultrasonography
Introduction
At present, coronary catheterizations and interventions are more often performed using the radial artery approach than by the previously more popular femoral artery approach due to the lower incidence of both local and systemic complications. Therefore, the radial approach has received a Class IA recommendation in the European Society of Cardiology (ESC) guidelines for interventional treatment of acute coronary syndromes, including STEMI and other forms of ischaemic heart disease.1,2 Although a large number of studies concerning the radial approach have been published, many questions remain unanswered. One of them is the method of choice for routine clinical use when evaluating radial artery occlusion (RAO), which is the most common local complication of the transradial procedure.
Palpation of the radial artery, which is commonly used, is not a reliable method. Non-palpable pulsation after a transradial catheterization is a strong indicator of RAO; nevertheless, a palpable pulse does not exclude the presence of RAO due to the coexistence of a collateral blood supply. In clinical trials, the patency or occlusion of the radial artery has mostly been determined using the reverse Barbeau test (RBT) or duplex ultrasonography. No study has directly compared DUSG and RBT in detecting radial artery patency or occlusion after transradial coronary procedures.
Methods
The primary aim of this study was to perform a comparison of the RBT and DUSG and investigate the possibility of using the RBT as the standard detection method of RAO. In addition, we aimed to evaluate radial artery compression time, the incidence of post-procedural haematomas, and other local complications at the puncture site.
Reverse Barbeau test
The RBT employs a pulse oximeter and displays the plethysmographic waveform using a sensor placed on the thumb of the hand being examined. First, both the radial and ulnar arteries are compressed simultaneously until the plethysmographic wave is lost. Then, the pressure on the radial artery is released, and the shape of the plethysmographic wave is evaluated. There are four types of waveforms: (A) no change in shape or amplitude (no dumping); (B) light dumping; (C) loss of the wave followed by its reappearance within 2 min; and (D) permanent loss of the wave. The presence of type A, B, and C waveforms demonstrate the patency of the radial artery (Figure 1).3
Figure 1.
The reverse Barbeau test demonstrating a patent radial artery after transradial catheterization. After the removal of the compression device, which had been placed on the radial artery, blood saturation and the corresponding plethysmographic wave were normal (left). During ulnar compression, the wave and blood saturation do not change, indicating patency of the radial artery (right).
Duplex ultrasonography
Duplex ultrasonography allows structural visualization of the radial artery and evaluation of blood flow using colour and pulsed Doppler imaging (Figure 2).4 Absence of flow indicates an RAO. This method is considered the gold standard for arterial flow assessment, and when there is a suspicion of an RAO then DUSG should be performed to confirm the finding.5
Figure 2.

Duplex ultrasonography in the detection of radial artery patency. Colour-coded blood flow in a patent radial artery (in red) and the corresponding typical triphasic curve visualized using pulsed Doppler.
After signing informed consent, patients indicated to transradial coronary catheterization in our same-day discharge program were enrolled in the study. The only exclusion criteria were refusal to participate in the study and warfarin treatment with an international normalized ratio (INR) > 2.5. Before the transradial procedure, all patients underwent a standard Barbeau test and the RBT; additionally, a palpation examination was used to assess the patency of the radial and ulnar arteries in the access arm; DUSG was not performed prior to the catheterization procedure.
A TR Band ® (Terumo, Japan) (TB) compression device was used to compress the radial artery puncture site after the procedure. During TB decompression, the aim was to achieve and maintain patent haemostasis. The principle involves a gradual reduction in radial artery compression to the point that it prevents bleeding but allows distal perfusion through the artery at the same time. Therefore, a careful balancing of the compression rate between complete (i.e. occlusive) haemostasis on the one hand and bleeding, on the other hand, is required. In detail, immediately after the patient brought from the Cath lab to the observation unit, the TB pressure being applied on the radial artery access site was gradually reduced until a simultaneous recording of the pulse oximeter plethysmographic wave, during ulnar artery compression indicated the presence of blood flow in the radial artery (Figure 3). If there was bleeding, TB pressure was again increased to the point where bleeding stopped. Subsequent decompression to the lowest possible level was performed every 20 min until the TB pressure was zero (i.e. no compression).
Figure 3.
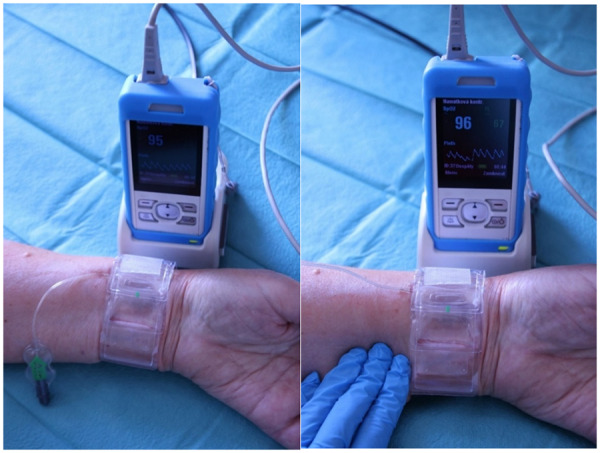
The use of the reverse Barbeau test in the detection of patent haemostasis during radial compression with a TR Band device. When the TR Band is in use, normal oxygen saturation and a triphasic plethysmographic wave are usually detected (left). When applying manual compression on the ulnar artery, the persistence of the curve indicates patent haemostasis (right).
All patients underwent both DUSG and the RBT before discharge, which was usually 4 h after a diagnostic coronarography and 6 h after PCI. If an RAO was detected, an additional 1-h period of ulnar artery compression with the same TB was used to facilitate the recanalization of the radial artery.
Data about history, antithrombotic medication, procedure characteristics, radial artery compression time, and local complications were recorded in all patients. Haematomas were evaluated according to the EASY classification.6 Grade 1 haematomas (surface haematomas ≤ 5 cm in length) were not recorded due to their negligible clinical significance.
Standard statistics were applied and parameters were described using absolute and relative frequencies or median (5–95 percentile range).
Results
Five hundred patients from our same-day discharge program (mean age 65 ± 9.4 years; 148 females) were enrolled in the study (Table 1). Of those, 379 (75.8%) underwent a diagnostic selective coronary angiography (CAG), and 121 (24.2%) underwent a subsequent percutaneous coronary intervention (PCI). Haemodynamic testing of borderline coronary lesions using fractional flow reserve measurement (FFR) was performed in 40 (8%) patients (Table 2).
Table 1.
Baseline clinical characteristics of the patients
| Characteristics | Patients (n = 500) |
|---|---|
| Female | 148 (29) |
| Age (years) | 65 ± 9.4 |
| BMI (kg/m2) | 29.9 ± 12.9 |
| Hypertension | 359 (71.8) |
| Hypercholesterolaemia | 348 (69.6) |
| Diabetes | 116 (23.2) |
| Smoker | 96 (19.2) |
| Prior smoker | 131 (26.2) |
| Prior myocardial infarction | 85 (17.0) |
| Prior PCI | 101 (20.2) |
| Prior CABG | 28 (5.6) |
| Prior ipsilateral transradial CAG | 119 (23.8) |
Parameters are described by absolute (relative, %) frequencies or median (5–95 percentile range).
BMI, body mass index; CABG, coronary artery bypass grafting; CAG, selective coronary angiography; PCI, percutaneous coronary intervention.
Table 2.
Patient procedure characteristics
| Procedures | Patients (n = 500) |
|---|---|
| CAG | 500 (100) |
| CAG with PCI | 121 (24.2) |
| BMS implantation | 35 (28.9) |
| DES implantation | 81 (67.0) |
| DEB only | 5 (4.1) |
| FFR | 40 (8.0) |
| LVG | 38 (7.6) |
Parameters are described by absolute (relative, %) frequencies.
BMS, bare-metal stent; CAG, selective coronary angiography; DEB, drug-eluting balloon; DES, drug-eluting stent; FFR, fractional flow reserve; LVG, left ventriculography; PCI, percutaneous coronary intervention.
The numbers of patients on concomitant oral antiplatelet or anticoagulant therapy are shown in Table 3. Direct anticoagulants were used in 9 patients (1.8%), 4 were taking apixaban, 3 rivaroxaban, and 2 dabigatran. None of these patients took a morning dose on the day of the procedure. All patients enrolled in the study received 5000 units of unfractionated heparin (UFH) intra-arterially immediately following a successful radial artery puncture and sheath introduction. In 8 (1.2%) patients undergoing PCI, the UFH dose was increased peri-procedurally based on their weight and procedure complexity; UFH increases were at the discretion of the operator. There were no thrombotic complications during coronary procedures relative to the heparin dosing schedule.
Table 3.
Concomitant antiplatelet and anticoagulation therapy prior to the procedure
| Antithrombotic therapy | Patients (n = 500) |
|---|---|
| Acetylsalicylic acid (100 mg daily) | 389 (77.8) |
| Clopidogrel (75 mg daily) | 158 (31.6) |
| Ticagrelor (90 mg twice a day) | 9 (1.8) |
| Warfarin (INR 2.0–2.5) | 36 (7.2) |
| DOACs | 9 (1.8) |
Parameters are described by absolute (relative, %) frequencies.
DOACs, direct oral anticoagulants; INR, international normalized ratio.
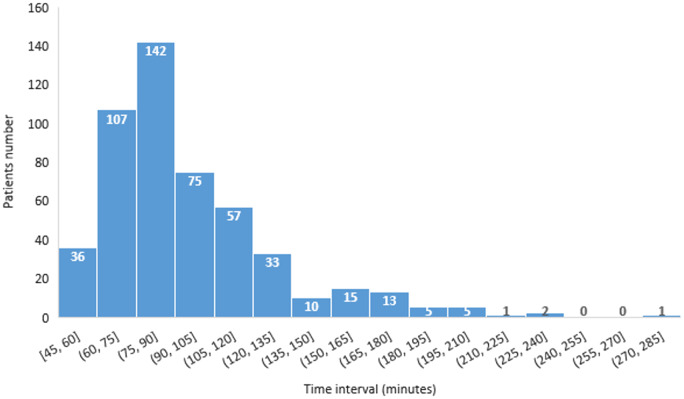
The mean total radial artery compression time in the study was 97.8 ± 33.7 min. Compression times and their distribution in the study cohort are shown in Figure 4.
Figure 4.
Distribution of radial artery compression time.
The ability to detect patency and complete radial artery occlusion was comparable between the RBT and DUSG. Both methods detected a complete occlusion in two same subjects (0.4%) and demonstrated radial artery patency in 495 (99.0%) subjects. In three (0.6%) patients, RBT detected a patent artery (i.e. the absence of a type D curve) while DUSG showed incomplete RAO, with traces of blood flow on the colour Doppler imaging but a significant reduction of flow velocities and loss of the typical triphasic wave in pulse Doppler imaging. Both patients with a complete RAO were asymptomatic; subsequent attempts to recanalize their radial artery by applying 60-min of ulnar compression were unsuccessful. This was confirmed using both RBT and DUSG.
The incidence of puncture site haematomas was low. There were only EASY Grade II subcutaneous haematomas, clinically insignificant, and asymptomatic (Table 4).
Table 4.
Incidence of local superficial haematomas (EASY classification)
| Haematoma grade | II (˃ 5 but ≤10 cm) | III (˃ 10 cm) | IV (forearm) | V (arm) |
|---|---|---|---|---|
| Number of pts | 27 (5.4) | 0 | 0 | 0 |
Parameters are described by absolute (relative, %) frequencies, N = 500 patients (pts). Minimal haematomas (Grade I) were not considered clinically relevant, thus not reported.
Other local complications included two (0.4%) minor dissections and one (0.2%) minor perforation of the radial artery. One of the dissections was detected angiographically at the beginning of the coronarography but did not prevent the procedure from being completed, and no subsequent haematoma developed. The other dissection was detected by DUSG after the procedure, and an EASY grade II haematoma developed. In both cases, the radial artery remained patent. Concerning the case of the minor perforation, despite the extravasation of blood detected angiographically at the beginning of the examination, the procedure was completed with no other difficulties; there was no angiographic evidence of extravasation at the end of the procedure and no subsequent haematoma. The artery was confirmed patent at the time of the patient’s dismissal.
Discussion
A radial artery occlusion is almost always asymptomatic when there is an intact ulnar artery, and the palmar arches are patent. Nevertheless, there are several important reasons to minimize the incidence of RAO, including keeping the potential to use the same radial artery for repeated catheterizations, utilizing the artery as a free arterial graft for a bypass operation, or as an arteriovenous fistula in dialyzed patients.
The incidence of RAO varies widely among studies (from <1% to 33%). A large meta-analysis of 66 studies with more than 31 000 patients reported the incidence of 7.7% at 24 h and 5.5% at 7 days.7
The following factors and methods have been identified in RAO prevention:
Non-occlusive haemostasis of the radial artery
Non-occlusive (patent) haemostasis is one of the most important methods currently used to reduce the risk of RAO after the catheterization. In a study published in 2007, the absence of radial artery flow during compression increased the risk of radial artery occlusion.8 The fact that patent haemostasis reduces the incidence of RAO was confirmed in the PROPHET study. The patients were randomized to conventional radial artery compression or non-occlusive haemostasis, with subsequent assessment of arterial patency using the Barbeau test after 24 h. The incidence of RAO in the patent haemostasis group was significantly lower compared to the conventional compression group (5% vs. 12%, p < 0.05).9
Our approach to patent haemostasis in this study was described in the Methods section.
Short and gentle compression
To reduce the incidence of an RAO, it is very important to keep the compression period as short as possible and the compression manoeuvre as gentle as possible. In a study published in 2012, a 6-h radial compression with TB more than doubled the incidence of both early (after 24 h) and late (after 30 days) occlusions compared to a shorter 2-h compression (12% vs. 5.5%, P = 0.025, and 8.5% vs. 3.5%, P = 0.035, respectively). When patency status at the time of compression initialization was added to a logistic regression analysis, occlusive haemostasis was the only independent predictor of late RAO (odds ratio 13.1, P = 0.001).10
In the CRASOC I-III studies, with over 3600 patients enrolled, gentle (with 10 mL of air) and short (1.5 h) radial compression with a TB resulted in a lower incidence of RAO (2.3%) compared to less gentle (13 mL) and longer (4 h) compression (9.4%).11
In our study, the compression time was <90 min in 285 (57%) patients and ≤120 min in 417 (83%) patients.
The importance of the outer sheath diameter to inner radial artery diameter ratio
Several studies have shown a positive correlation between a smaller sheath size and a lower incidence of RAO. In a randomized study by Dahm et al., the 5 Fr transradial sheath group of patients had an RAO incidence of 1.1% compared to 5.9% in the 6 Fr sheath group (P = 0.05).12 In another randomized and multicentre study with nearly 2000 patients, the use of a thin-walled 6 Fr sheath (outer diameter 2.46 mm) was compared to a standard 5 Fr sheath (outer diameter 2.29 mm). The authors concluded that the thin-walled 6 Fr sheath was associated with a lower event rate of RAO, although non-inferiority to the standard 5 Fr sheath was not established, due to a lower than expected rate of RAO in the 5 Fr sheath group.13
In our study, the choice of a sheath size was left to the operator’s discretion. Thin-walled 5 Fr sheaths were used in 239 patients (47.8%), standard 5 Fr sheaths were used in 131 (26.2%), and thin-walled 6 Fr sheaths were used in 130 patients (26.0%). One RAO was associated with the use of the standard 5 Fr sheath and the second RAO with the thin-walled 6 Fr sheath.
Transradial PCI can be performed also with larger than 6 Fr sheaths. In study with 175 selected patients, the usage of 7 Fr sheaths in transradial complex PCI was feasible and associated with 7.3% RAO after 6 months.14 Even transradial balloon aortic valvuloplasty with 9 Fr introducer is possible in selected patients. However, this approach is associated with 20% 1 month RAO as reported in Tumscitźs pilot study.15
Adequate periprocedural anticoagulation
An insufficient dose of periprocedural heparin has been confirmed as another major risk factor for RAO. A study published in 1996 showed that omitting UFH during transradial selective coronarography was associated with a 71% incidence of RAO, a dose of 2000 to 3000 units reduced this incidence to 24% and a dose of 5000 units to 4.3% (P < 0.05).16
Administration of a weight-adjusted UFH dose (50 units/kg) has similar efficacy compared to a fixed dose of 5000 units and reduces the time to haemostasis.17 Similarly, no difference in efficacy has been found between intra-arterial and intravenous UFH application (RAO rates of 3.2% vs. 4%, P > 0.6).18
In a randomized study from our centre with 465 patients, the incidence of RAO detected by DUSG was 2.9% after 5000 UFH units and 5.9% after 2000 UFH units (P = 0.17). Nevertheless, a subsequent 1-h compression of the ulnar artery reduced the incidence of RAO to 0.8% and 4.1% (P = 0.03), respectively.19 The application of this simple, non-pharmacological method led to the recanalization of the radial artery in 71% of patients with a higher dose of UFH.
In this study, 5000 UFH units were initially used in all patients. Extra heparin was only given to a few patients, i.e., some of those who underwent PCI after the diagnostic procedure.
Prophylactic ipsilateral ulnar compression
To further reduce the risk of RAO, it is feasible to apply ipsilateral ulnar compression concurrently with standard radial artery compression. This method was tested in the large PROPHET-II study in which 3000 patients were randomized to standard patent haemostasis or prophylactic ulnar compression. The incidence of RAO was evaluated using the RBT test at 24 h and at 30 days. At both time points, prophylactic ulnar compression resulted in significantly fewer cases of RAO (1% vs. 4.3%, P < 0.0001, and 0.9% vs. 3%, P = 0.0001, respectively).20 This method was not used in this study.
Our study showed that the use of the RBT for radial artery patency confirmation after transradial cardiac catheterization is very effective. It is not necessary to perform DUSG routinely, which is more time demanding and requires advanced instrumentation and skills. The reverse Barbeau test has the potential to be the method of choice for detecting radial artery patency or occlusion.
The very low incidence of complete radial artery occlusion in our study (0.4%) stems from our long-standing and well-established standardized operating protocol, which is based on the concept of optimizing the approach in RAO prevention.5
From our point of view, data from this study provide important insights into the successful management of radial access sites in patients undergoing transradial coronary catheterizations and interventions.
Study limitations
The presented data are from a single-centre only, and therefore, the results should be interpreted and generalized with caution. Furthermore, the RAO number was very low and did not allow a more detailed comparison of the two methods in actual RAO cases.
Conclusions
The reverse Barbeau test and duplex ultrasonography were comparable in the detection of radial artery patency and occlusion. The incidence of radial artery occlusion in our study was extremely low. The RBT with a pulse oximeter and plethysmographic waveform is much faster and easier to use in a routine practice compared to DUSG. Thanks to its simplicity, the RBT has the potential to be used as the first method of detection of radial occlusion after coronary catheterizations.
Funding
This paper was published as part of a supplement financially supported by the Cardiovascular Research Program of the Charles University ‘Progres Q38’ and the Ministry of Health of the Czech Republic Conceptual Development of Research Organization [Faculty Hospital in Pilsen (FNPl), 00669806].
Data availability statement
Data available on request.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, Collet J-P, Kristensen SD, Aboyans V, Baumbach A, Bugiardini R, Coman IM, Delgado V, Fitzsimons D, Gaemperli O, Gershlick AH, Gielen S, Harjola V-P, Katus HA, Knuuti J, Kolh P, Leclercq C, Lip GYH, Morais J, Neskovic AN, Neumann F-J, Niessner A, Piepoli MF, Richter DJ, Shlyakhto E, Simpson IA, Steg PG, Terkelsen CJ, Thygesen K, Windecker S, Zamorano JL, Zeymer U, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Chettibi M, Hayrapetyan HG, Metzler B, Ibrahimov F, Sujayeva V, Beauloye C, Dizdarevic-Hudic L, Karamfiloff K, Skoric B, Antoniades L, Tousek P, Terkelsen PJ, Shaheen SM, Marandi T, Niemelä M, Kedev S, Gilard M, Aladashvili A, Elsaesser A, Kanakakis IG, Merkely B, Gudnason T, Iakobishvili Z, Bolognese L, Berkinbayev S, Bajraktari G, Beishenkulov M, Zake I, Lamin HB, Gustiene O, Pereira B, Xuereb RG, Ztot S, Juliebø V, Legutko J, Timóteo AT, Tatu-Chiţoiu G, Yakovlev A, Bertelli L, Nedeljkovic M, Studenčan M, Bunc M, García de Castro AM, Petursson P, Jeger R, Mourali MS, Yildirir A, Parkhomenko A, Gale CP; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 2. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Wijns W, Glineur D, Aboyans V, Achenbach S, Agewall S, Andreotti F, Barbato E, Baumbach A, Brophy J, Bueno H, Calvert PA, Capodanno D, Davierwala PM, Delgado V, Dudek D, Freemantle N, Funck-Brentano C, Gaemperli O, Gielen S, Gilard M, Gorenek B, Haasenritter J, Haude M, Ibanez B, Iung B, Jeppsson A, Katritsis D, Knuuti J, Kolh P, Leite-Moreira A, Lund LH, Maisano F, Mehilli J, Metzler B, Montalescot G, Pagano D, Petronio AS, Piepoli MF, Popescu BA, Sádaba R, Shlyakhto E, Silber S, Simpson IA, Sparv D, Tavilla G, Thiele H, Tousek P, Van Belle E, Vranckx P, Witkowski A, Zamorano JL, Roffi M, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Sousa-Uva M, Simpson IA, Zamorano JL, Pagano D, Freemantle N, Sousa-Uva M, Chettibi M, Sisakian H, Metzler B, İbrahimov F, Stelmashok VI, Postadzhiyan A, Skoric B, Eftychiou C, Kala P, Terkelsen CJ, Magdy A, Eha J, Niemelä M, Kedev S, Motreff P, Aladashvili A, Mehilli J, Kanakakis I-G, Becker D, Gudnason T, Peace A, Romeo F, Bajraktari G, Kerimkulova A, Rudzītis A, Ghazzal Z, Kibarskis A, Pereira B, Xuereb RG, Hofma SH, Steigen TK, Witkowski A, de Oliveira EI, Mot S, Duplyakov D, Zavatta M, Beleslin B, Kovar F, Bunc M, Ojeda S, Witt N, Jeger R, Addad F, Akdemir R, Parkhomenko A, Henderson R; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 3. Barbeau GR, Arsenault F, Dugas L, Simard S, Larivière MM.. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heat J 2004;147:489–493. [DOI] [PubMed] [Google Scholar]
- 4. Peruga JP, Peruga JZ, Kasprzak JD, Kręcki R, Jankowski Ł, Zając P, Plewka M.. Ultrasound evaluation of forearm arteries in patients undergoing percutaneous coronary intervention via radial artery access: results of one-year follow-up. Kardiol Pol 2015;73:502–510. [DOI] [PubMed] [Google Scholar]
- 5. Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, Gilchrist IC, Saito S, Hahalis GN, Ziakas A, Louvard Y, Montalescot G, Sgueglia GA, van Leeuwen MAH, Babunashvili AM, Valgimigli M, Rao SV, Bertrand OF; RAO International Group. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv 2019;12:2235–2246. [DOI] [PubMed] [Google Scholar]
- 6. Bertrand OF, Larose E, Rodés-Cabau J, Gleeton O, Taillon I, Roy L, Poirier P, Costerousse O, Larochellière RD.. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J 2009;157:164–169. [DOI] [PubMed] [Google Scholar]
- 7. Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, Ratib K, Large A, Fraser D, Nolan J, Mamas MA.. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanmartin M, Gomez M, Rumoroso JR, Sadaba M, Martinez M, Baz JA, Iniguez A.. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv 2007;70:185–189. [DOI] [PubMed] [Google Scholar]
- 9. Pancholy S, Coppola J, Patel T, Roke-Thomas M.. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv 2008;72:335–340. [DOI] [PubMed] [Google Scholar]
- 10. Pancholy SB, Patel TM.. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter Cardiovasc Interv 2012;79:78–81. [DOI] [PubMed] [Google Scholar]
- 11. Dangoisse V, Guédès A, Chenu P, Hanet C, Albert C, Robin V, Tavier L, Dury C, Piraux O, Domange J, Jourdan K, Bihin B, Schroeder E.. Usefulness of a gentle and short hemostasis using the transradial band device after transradial access for percutaneous coronary angiography and interventions to reduce the radial artery occlusion rate (from the prospective and randomized CRASOC I, II, and III studies). Am J Cardiol 2017;120:374–379. [DOI] [PubMed] [Google Scholar]
- 12. Dahm JB, Vogelgesang D, Hummel A, Staudt A, Völzke H, Felix SB.. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv 2002;57:172–176. [DOI] [PubMed] [Google Scholar]
- 13. Aminian A, Saito S, Takahashi A, Bernat I, Jobe RL, Kajiya T, Gilchrist IC, Louvard Y, Kiemeneij F, Van Royen N, Yamazaki S, Matsukage T, Rao SV.. Comparison of a new slender 6 Fr sheath with a standard 5 Fr sheath for transradial coronary angiography and intervention: RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT), a randomised multicentre trial. EuroIntervention 2017;13:e549–e556. [DOI] [PubMed] [Google Scholar]
- 14. Tumscitz C, Pirani L, Tebaldi M, Campo G, Biscaglia S.. Seven french radial artery access for PCI: a prospective single-center experience. Int J Cardiol 2014;176:1074–1075. [DOI] [PubMed] [Google Scholar]
- 15. Tumscitz C, Campo G, Tebaldi M, Gallo F, Pirani L, Biscaglia S.. Safety and feasibility of transradial mini -invasive balloon aortic valvuloplasty: a pilot study. JACC Cardiovasc Interv 2017;10:1375–1377. [DOI] [PubMed] [Google Scholar]
- 16. Spaulding C, Lefèvre T, Funck F, Thébault B, Chauveau M, Ben Hamda K, Chalet Y, Monségu H, Tsocanakis O, Py A, Guillard N, Weber S.. Left radial approach for coronary angiography: results of a prospectove study. Cathet Cardiovasc Diagn 1996;39:365–370. [DOI] [PubMed] [Google Scholar]
- 17. Schiano P, Barbou F, Chenilleau MC, Louembe J, Monsegu J.. Adjusted weight anticoagulation for radial approach in elective coronarography: the AWARE coronarography study. EuroIntervention 2010;6:247–250. [PubMed] [Google Scholar]
- 18. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol 2009;104:1083–1085. [DOI] [PubMed] [Google Scholar]
- 19. Bernat I, Bertrand OF, Rokyta R, Kacer M, Pesek J, Koza J, Smid M, Bruhova H, Sterbakova G, Stepankova L, Costerousse O.. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol 2011;107:1698–1701. [DOI] [PubMed] [Google Scholar]
- 20. Pancholy SB, Bernat I, Bertrand OF, Patel TM.. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv 2016;9:1992–1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.