With the outbreak of the coronavirus disease 2019 (COVID‐19) pandemic, 1 Italy and in particular the largest Northern regions became the disease epicentre, with a dramatic rise in infections and deaths in March 2020. 2 Starting from the 11 March 2020, Italy was locked down and extraordinary measures were implemented to limit the spread of the infection: social distancing, self‐isolation/quarantine, prohibition of movements unless for necessity and closure of all non‐essential shops. The healthcare system was put under pressure and healthcare professionals faced a variety of changes and challenges, which also involved the conduct of clinical trials. 3 , 4
The authorities released guidelines on how to manage clinical trials during the pandemic and experts published recommendations on how to handle patients with cancer. 5 , 6 , 7 , 8 , 9 , 10
However, it was a principal investigator (PI) responsibility to adopt the most suitable measures to safeguard staff and patients, and a sponsor duty to monitor and implement measures to prioritise participants’ safety and data validity. To get insights into the actual changes that occurred at trials sites, we surveyed the PIs of five Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) experimental clinical trials for adult patients with leukaemia and we analysed the number of new enrolments.
Methods
Surveys covered four main topics, i.e. changes in the ongoing trials, measures implemented to mitigate the spreading of the infection to the staff, implications for patients and study staff, and study‐specific questions.
We queried the PIs of the GIMEMA centres, as detailed in Data S1, participating in five experimental trials (Table S1), namely LAL2116 (n = 26), LAL2317 (n = 44), LLC1114 (n = 35), LLC1518 (n = 26) and CML1415 (n = 52).
Surveys were launched on the 23 April 2020 and were managed using REDCap electronic data capture tools hosted at GIMEMA. 11 , 12 Results were exported on 22 May 2020 and analysed by r software. 13
In the three clinical trials open to enrolment (i.e. LAL2317, LLC1518 and CML1415), we analysed the number of new enrolments.
Results and discussion
We collected 136 surveys (74·3% response rate). The list of respondent PIs is provided in Table S1 and their geographical distribution is depicted in Figure S1.
The main changes in the management of patients occurred in the conduct of visits. Indeed, the latter were converted into telephone/video visits, as answered by 56·3% of the PIs, postponed (52·6%) or cancelled (25·4%); home nursing was only marginally (6%) implemented. These alternatives, although preventing the discontinuation of the trial, are not devoid of risks that may derive from delays in the scheduled visits, differences with in‐person visits and incomplete visit data.
Reassuringly, halt of trial sites was a rare event (3·8% of the PIs) and trial site closure was not reported (Fig 1A).
Fig 1.
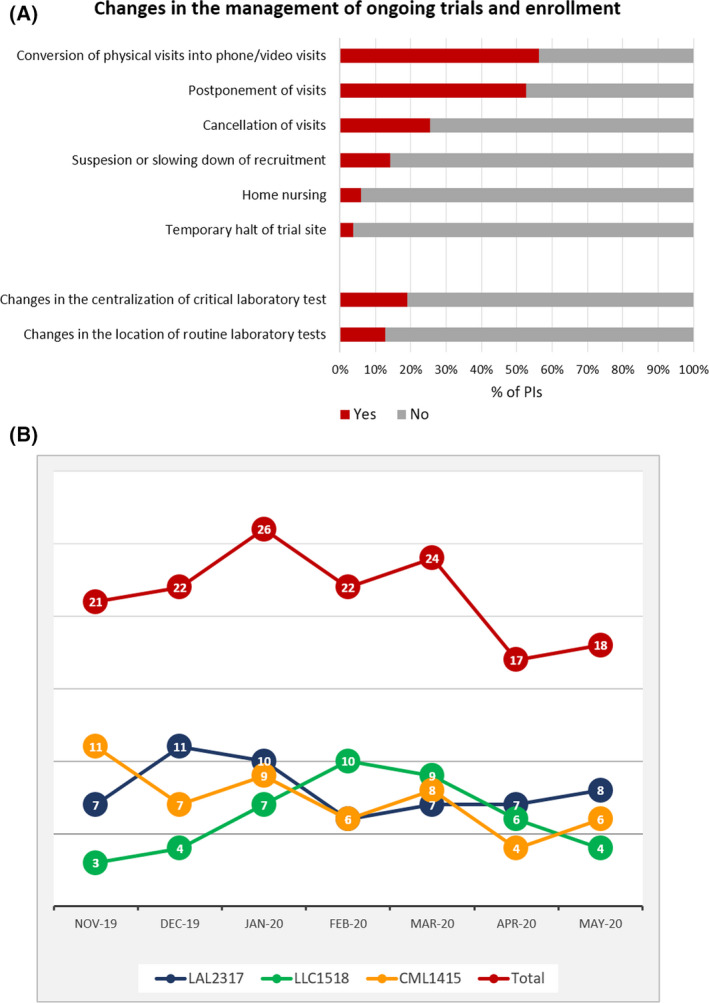
(A) Main changes in ongoing trials and impact on laboratory tests and clinical procedures. (B) Number of enrolled patients from November 2019 to May 2020 in the currently enrolling clinical trials.
Moderate changes occurred in the execution of laboratory tests, with 20% of the PIs declaring that the centralisation of laboratory tests was not carried out and 12·7% who reported a transfer of laboratory tests to a different location (Fig 1A). The PIs commented that the service was preserved mainly by storing the samples or performing the tests locally. Notwithstanding, these different procedures may result in a lack of uniformity in sample processing and affect assay results.
In the section dedicated to protocol‐specific questions, we queried about minimal residual disease quantification, which according to a low number of respondents (8% of the LAL2317 PIs and 17·4% of the LAL2116 PIs) was not regularly performed. A discontinuation of such a pivotal activity may lead to inadequate disease monitoring and compromise the achievement of the primary objective of the studies. Nevertheless, to draw definitive conclusions, it is warranted to go beyond aggregated data and analyse patients’ data: to this purpose, GIMEMA is collecting COVID‐19‐related information in dedicated ‘Case Report Forms’ (CRFs).
Alarming data came from the analysis of new enrolments: indeed, starting from April, we registered a decline of 25% that continued also in May, when restrictive measures were released. These data indicate that the effect of constraints may last beyond the months of lockdown (Fig 1B). The PIs ascribed this reduction to the stay‐at‐home measures that produced a reduction of first visits and diagnostic tests. This issue may have an impact on sample size and may introduce selection biases towards the patients with severer symptoms that require access to hospitals.
With regards to the workload, almost half of the PIs judged as ‘mild’ the burden the study staff had to bear. Performing visits was reported as the most frequent problem the study‐staff had to face (42·6% of the PIs), followed by completing CRFs (22·8%) and complying with the protocol (15·4%, Fig 2A), hence indicating the likelihood of missing/delayed data and protocol deviations.
Fig 2.
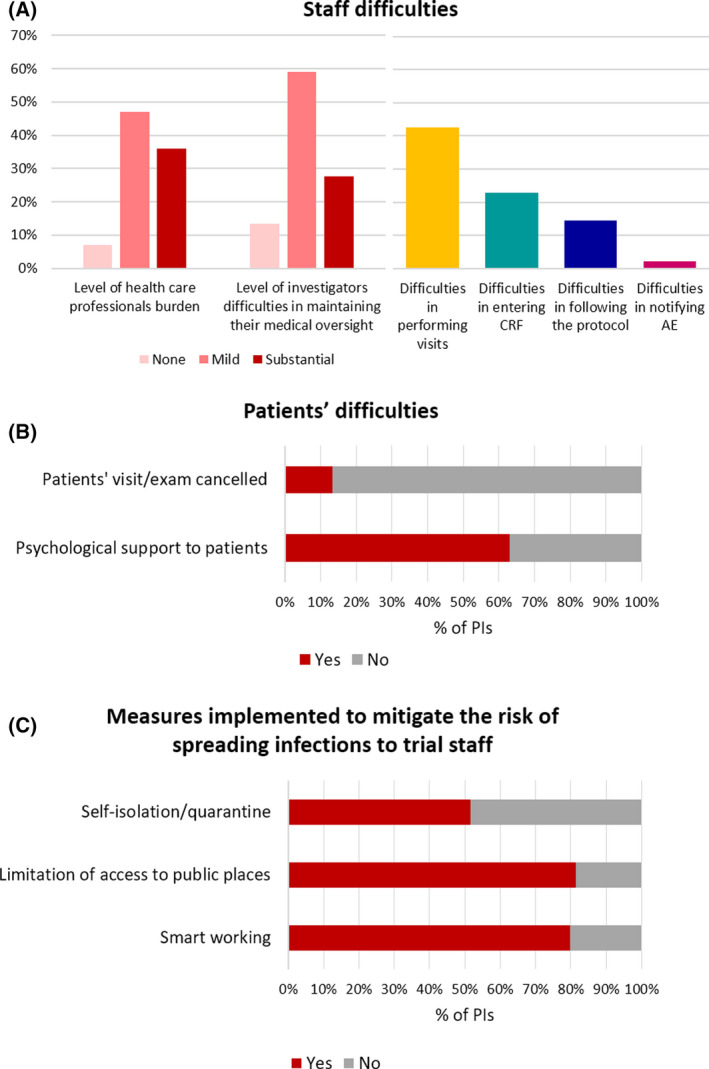
(A) Staff difficulties. (B) Patients’ difficulties. (C) Measures implemented to mitigate the risk of spreading infection to trial staff.
From the patients’ standpoint, 13·4% of the PIs reported the cancellation of visits by patients, thus indicating some difficulties/reluctances in visiting hospitals. This occurred especially in the trials for chronic leukaemias, thus mirroring an effect of the disease status. Notably, even during this emergency, psychological support was preserved (Fig 2B).
The measures to moderate the spread of infections to study staff included: limitation of access to public places (81·5% of the PIs), smart‐working for data managers (79·9%), self‐isolation/quarantine of the staff (51·5%, Fig 2C).
The comparison of the regions with the highest number of deceased patients with the others revealed that the strategies adopted were homogenous. Detailed results are described in Data S1 and Table S3.
Early in May, the American Society of Clinical Oncology published the results of a survey on the conduct of oncology trials. 14 The authors received 32 surveys and reported that the main changes occurred in patients’ ability to visit their sites and among the main challenges they listed telehealth, availability of ancillary services, discussion with sponsors, Clinical Research Organisations and Institutional Review Boards.
The present study surveyed a large number of PIs and focussed on the status of clinical trials for adult patients with leukaemia during the COVID‐19 pandemic. In the ongoing trials, our present findings warn of the major risks and possible effects on protocol results, thus allowing to get prepared for protocol amendments and allowing the adoption of the most suitable corrections. Looking to the future, the present study poses several questions about the management of upcoming trials: will we go back to normality or will we face a new normality? A new normality could entail the adoption of new risk assessment methodologies, closer and continuous monitoring, and alternative trial design. In this respect, an attractive alternative is represented by adaptive designs that ensure real‐time learning in order to guarantee that the trials are adequately powered to answer study objectives. 15
Disclosure of conflicts of interest
The authors do not have conflict of interest to disclose.
Author contributions
Alfonso Piciocchi designed the research, analysed data and wrote the manuscript; Monica Messina interpreted the results and wrote the manuscript; Stefano Soddu, Edoardo La Sala and Francesca Paoloni planned and designed the surveys; Giorgia Giuliani, Martina R. Marino, Claudia Petrelli, Livia Gorreo Renzulli collected and managed the surveys; Paola Fazi and Marco Vignetti designed the research and critically revised the manuscript.
Supporting information
Fig S1. Geographical distribution of participants.
Table S1. List of the GIMEMA clinical trials included in the study.
Table S2. List of principal investigators (PIs) participating in the survey.
Table S3. Survey questions and results.
Data S1. Supplementary methods.
Acknowledgements
We thank Prof. Robin Foà who encouraged this study and all the PIs who devoted time to complete the survey during an unprecedented medical challenge.
References
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health. Lancet. 2020;395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Characteristics of SARS‐CoV‐2 patients dying in Italy. Report based on available data on 14 May 2020. Available from: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report‐COVID‐2019_14_may_2020.pdf.
- 3. McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135. DOI: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 4. Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard‐Lucey VM. Impact of COVID‐19 on oncology clinical trials. Nat Rev Drug Discov. 2020;19:376–7. DOI: 10.1038/d41573-020-00093-1. [DOI] [PubMed] [Google Scholar]
- 5. AIFA Comunicazione del 12.03.2020 “Gestione degli studi clinici in Italia in corso di emergenza COVID‐19 (coronavirus disease 19)”. Available from: https://www.aifa.gov.it/documents/20142/871583/Comunicato_gestione_studi_clinici_in_emergenza_COVID‐19_12.03.2020.pdf.
- 6. European Medicines Agency .EMA Guidance on the Management of Clinical Trials during the COVID‐19 (Coronavirus) pandemic Version 1 (20/03/2020). Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol‐10/guidanceclinicaltrials_covid19_en.pdf.
- 7. United States Food and Drug Administration .FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID‐19 Public Health Emergency. Guidance for Industry, Investigators, and Institutional Review Boards. Available from: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/fda‐guidance‐conduct‐clinical‐trials‐medical‐products‐during‐covid‐19‐public‐health‐emergency.
- 8. von Lilienfeld‐Toal M, Vehreschild JJ, Cornely O, Pagano L, Compagno F, EHA Infectious Disease Scientific Working Group et al Frequently asked questions regarding SARS‐CoV‐2 in cancer patients‐recommendations for clinicians caring for patients with malignant diseases. Leukemia. 2020;34:1487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paul S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD et al Treating leukemia in the time of COVID‐19. Acta Haematol. 2020. [Online ahead of print]. DOI: 10.1159/000508199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W et al COVID‐19 in persons with haematological cancers. Leukemia. 2020;34:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R‐project.org/. [Google Scholar]
- 12. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L et al REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waterhouse DM, Harvey RD, Hurley P, Levit LA, Kim ES, Klepin HD et al Early impact of COVID‐19 on the conduct of oncology clinical trials and long‐term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol Pract. 2020. [Online ahead of print]. DOI: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 15. Kairalla JA, Coffey CS, Thomann MA, Muller KE. Adaptive trial designs: a review of barriers and opportunities. Trials. 2012;13:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Geographical distribution of participants.
Table S1. List of the GIMEMA clinical trials included in the study.
Table S2. List of principal investigators (PIs) participating in the survey.
Table S3. Survey questions and results.
Data S1. Supplementary methods.


