Abstract
Objective
To report the incidence of fever among patients who tested positive for SARS‐CoV‐2.
Methods
Retrospective cohort study of patients who tested positive for SARS‐CoV‐2 at a single centre. Temperature at time of testing and on repeat testing within 24 h were collected.
Results
At the time of testing, fever was detected (sensitivity) in 16 of 86 (19%; 95% confidence interval 11–28) episodes of positive tests for SARS‐CoV‐2. With repeat testing, fever was detected in 18 of 75 (24%; 95% confidence interval 15–35) episodes.
Conclusions
In an Australian hospital, screening for fever lacked sensitivity for detection of patients with SARS‐CoV‐2.
Keywords: COVID‐19, emergency medicine, fever, screening, temperature
Introduction
Coronavirus disease (COVID‐19), caused by the SARS‐CoV‐2 virus, is a potentially fatal disease of global public health concern. Fever has been reported to be a common clinical finding in COVID‐19, prompting widespread temperature screening across multiple sectors, including hospitals, office buildings and airports.
Fever screening has been driven by overseas data. For example, a meta‐analysis of 1995 COVID‐19 cases from China reported fever in 89%, with about 50% febrile at the time of hospital admission. 1 , 2 Fever was also reported in 64% of healthcare workers who tested positive in New York; 3 in 45% of imported COVID‐19 cases in Taiwan and 45% of patients with mild–moderate disease in Europe. 4 , 5
Essential resources are being allocated to temperature screening, including nursing staff at hospital entrances and investment in technology for mass screening. The present study aimed to report the incidence of fever among patients who tested positive for SARS‐CoV‐2 in an Australian hospital setting.
Methods
Setting
The study was conducted at an adult major referral hospital in metropolitan Melbourne, Victoria, Australia, with an annual ED attendance of approximately 65 000 patients.
Participants
All patients who underwent testing for SARS‐CoV‐2 from 9 March 2020 to 13 May 2020 were eligible for inclusion. Patients presenting for screening of COVID‐19 only were excluded as temperatures were not recorded. Episodes of repeat testing within a 24‐h period were excluded.
Design
This was a retrospective cohort study including all patients who presented to hospital and returned a positive test for SARS‐CoV‐2. The primary outcome was sensitivity of fever for a positive SARS‐CoV‐2 test result. Testing of nose and throat swab samples was performed using quantitative reverse‐transcription polymerase chain reaction. 6 Data were extracted for the outcome variables of body temperature at the time of testing (or closest available recording) and when repeated, the highest temperature within the next 24 h. Age, sex and mode of temperature measurement were also extracted. Based on Australian government guidelines, fever was defined as a body temperature of ≥38°C. 7
Analysis
Results were reported using proportions with 95% confidence intervals (CIs). All analyses were conducted using stata version 15 (College Station, TX, USA). The present study was performed as part of the COVED registry that was approved by The Alfred Hospital Research and Ethics Committee. 8
Results
There were 86 tests on 34 patients retained for analysis, of which 75 tests were repeated (Fig. 1). Included patients were aged 55 (standard deviation 15) years and 25 (73.5%) were of male sex. The primary indication (available for 68 episodes) was pneumonia (24; 35%), case contact (15; 22%), being in a moderate or high‐risk setting (16; 24%), symptoms (8; 12%), overseas travel (4; 6%) and advanced age (1; 1%). Most measurements were performed using temporal thermometers (53; 62%); 14 (16%) were measured from the bladder, 5 (6%) from the ear canal, 4 (5%) oral, 4 (5%) nasopharyngeal, 4 (5%) axillary and mode of measurement was not recorded for two episodes.
Figure 1.
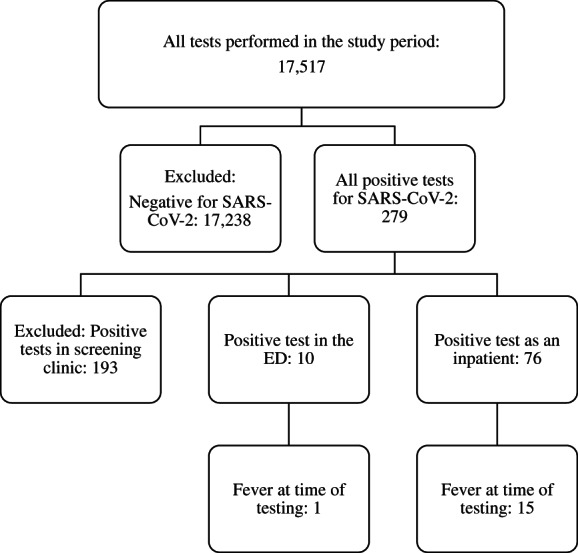
Selection of patients.
Fever at the time of testing (sensitivity) was detected in 16 (19%; 95% CI 11–28) episodes, while fever on repeat testing within 24 h was detected in 18 (24%; 95% CI 15–35) episodes (Fig. 2).
Figure 2.
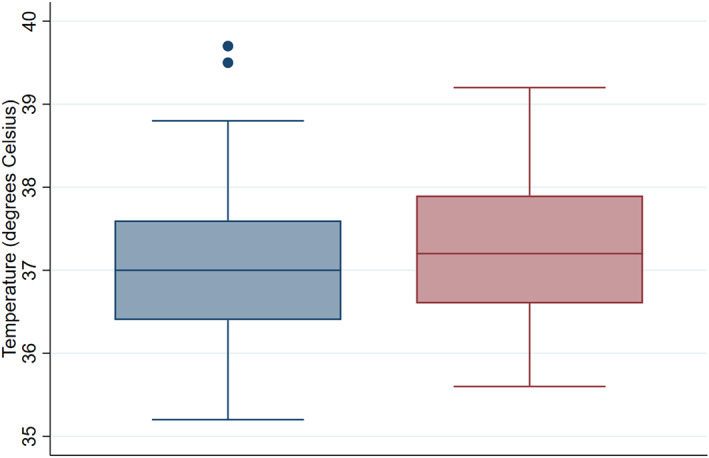
Body temperature at time of being tested positive for SARS‐CoV‐2 and highest temperature in subsequent 24 h. ( ) Time of positive test; (
) Time of positive test; ( ) highest temperature in subsequent 24 h.
) highest temperature in subsequent 24 h.
Discussion
In this single‐centre Australian study, fever was uncommon among hospital patients who tested positive for SARS‐CoV‐2. Even when attempting to account for variability in temperature by considering repeat testing, sensitivity did not improve to useful levels. Most measurements were of core temperature and screening using other methods could further reduce sensitivity.
This observation contrasts with overseas reports, where fever has been reported to be common. This possibly reflects differences in epidemiology of the pandemic in Australia, which has adopted liberal testing criteria and experienced a relatively low burden of critical illness. Our finding, that fever had a low sensitivity for SARS‐CoV‐2, questions any utility of widespread temperature screening. The sensitivity of fever also appears even lower in the initial stages of the illness (when tested in the ED) versus later during in the course of the illness (wards). Moreover, using fever as a screening tool for COVID‐19 may provide a false sense of security. While screening for fever may have alternative public health benefits, its value in excluding SARS‐CoV‐2 is limited in our population.
Our findings are consistent with previously published opinion. 9 , 10 The role of screening for fever at airports has also been questioned, with exit or entry screening with thermal scanners thought to be largely ineffective in controlling the spread of SARS‐CoV‐2. 11 Generic public health measures, such as self‐isolation when sick, physical distancing and contact tracing, are more likely to be effective than widespread temperature screening.
Acknowledgements
GMOR is currently a NHMRC Research Fellow at the National Trauma Research Institute, Alfred Hospital, Melbourne, Australia, leading the project titled: Maximising the usefulness and timeliness of trauma and emergency registry data for improving patient outcomes.
Competing interests
BM, GMOR and PAC are section editors for Emergency Medicine Australasia.
Biswadev Mitra, MBBS, MHSM, PhD, FACEM, Director of Emergency Medicine Research; Carl Luckhoff, MBBS, FACEM, Deputy Director; Rob D Mitchell, MBBS (Hons), BMedSc (Hons), MPH&TM, FACEM, Emergency Physician; Gerard M O'Reilly, MBBS, MPH, MBiostat, AStat, FACEM, PhD, Emergency Physician; De Villiers Smit, MBChB, FACEM, Director; Peter A Cameron, MBBS, MD, FACEM, Academic Director.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Li LQ, Huang T, Wang YQ et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J. Med. Virol. 2020; 92: 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clemency BM, Varughese R, Scheafer DK et al. Symptom criteria for COVID‐19 testing of heath care workers. Acad. Emerg. Med. 2020; 27: 469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu JY, Chen TJ, Hwang SJ. Analysis of imported cases of COVID‐19 in Taiwan: a nationwide study. Int. J. Environ. Res. Public Health 2020; 17: 3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lechien JR, Chiesa‐Estomba CM, Place S et al. Clinical and epidemiological characteristics of 1420 European patients with mild‐to‐moderate coronavirus disease 2019. J. Intern. Med. 2020; 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharfstein JM, Becker SJ, Mello MM. Diagnostic testing for the novel coronavirus. JAMA 2020; 323: 1437–8. [DOI] [PubMed] [Google Scholar]
- 7.Healthdirect. Fever 2020. [Cited 19 May 2020.] Available from URL: https://www.healthdirect.gov.au/fever
- 8. O'Reilly GM, Mitchell RD, Noonan MP et al. Informing emergency care for COVID‐19 patients: the COVID‐19 Emergency Department Quality Improvement Project protocol. Emerg. Med. Australas. 2020; 32: 511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bwire GM, Paulo LS. Coronavirus disease‐2019: is fever an adequate screening for the returning travelers? Trop. Med. Health 2020; 48: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Reilly GM, Mitchell RD, Rajiv P et al. Epidemiology and clinical features of emergency department patients with suspected COVID‐19: initial results from the COVID‐19 Emergency Department Quality Improvement Project (COVED‐1). Emerg. Med. Australas. 2020; 32: 638–45. [DOI] [PubMed] [Google Scholar]
- 11. Quilty BJ, Clifford S, Flasche S et al. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019‐nCoV). Eurosurveillance 2020; 25: 2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


