Abstract
There are several risk factors for worse outcomes in patients with coronavirus 2019 disease (COVID‐19). Patients with hypertension appear to have a poor prognosis, but there is no direct evidence that hypertension increases the risk of new infection or adverse outcomes independent of age and other risk factors. There is also concern about use of renin‐angiotensin system (RAS) inhibitors due to a key role of angiotensin‐converting enzyme 2 receptors in the entry of the SARS‐CoV‐2 virus into cells. However, there is little evidence that use of RAS inhibitors increases the risk of SARS‐CoV‐2 virus infection or worsens the course of COVID‐19. Therefore, antihypertensive therapy with these agents should be continued. In addition to acute respiratory distress syndrome, patients with severe COVID‐19 can develop myocardial injury and cytokine storm, resulting in heart failure, arteriovenous thrombosis, and kidney injury. Troponin, N‐terminal pro‐B‐type natriuretic peptide, D‐dimer, and serum creatinine are biomarkers for these complications and can be used to monitor patients with COVID‐19 and for risk stratification. Other factors that need to be incorporated into patient management strategies during the pandemic include regular exercise to maintain good health status and monitoring of psychological well‐being. For the ongoing management of patients with hypertension, telemedicine‐based home blood pressure monitoring strategies can facilitate maintenance of good blood pressure control while social distancing is maintained. Overall, multidisciplinary management of COVID‐19 based on a rapidly growing body of evidence will help ensure the best possible outcomes for patients, including those with risk factors such as hypertension.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, biomarkers, cardiac injury, COVID‐19, home blood pressure monitoring, hypertension, telemedicine
1. INTRODUCTION
The infectious disease caused by the new severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), COVID‐19, broke out in Wuhan, China, and spread to almost every country in the world. Millions of people have been infected, many have died, and everyday life has changed completely. The disease is accompanied by range of different symptoms (Figure 1). Rapidly accumulating data show that prognosis for patients with COVID‐19 is good in those with mild disease, but severe cases show relatively asymptomatic early progression followed by rapid worsening after symptom onset, culminating in acute respiratory distress syndrome (ARDS) and significant disease manifestations (Figure 2). The presence of SARS‐CoV‐2 has been detected in multiple organs on autopsy, including the pharynx, lungs, heart, liver, brain and kidneys, highlighting the multiorgan tropism of this virus. 1
Figure 1.
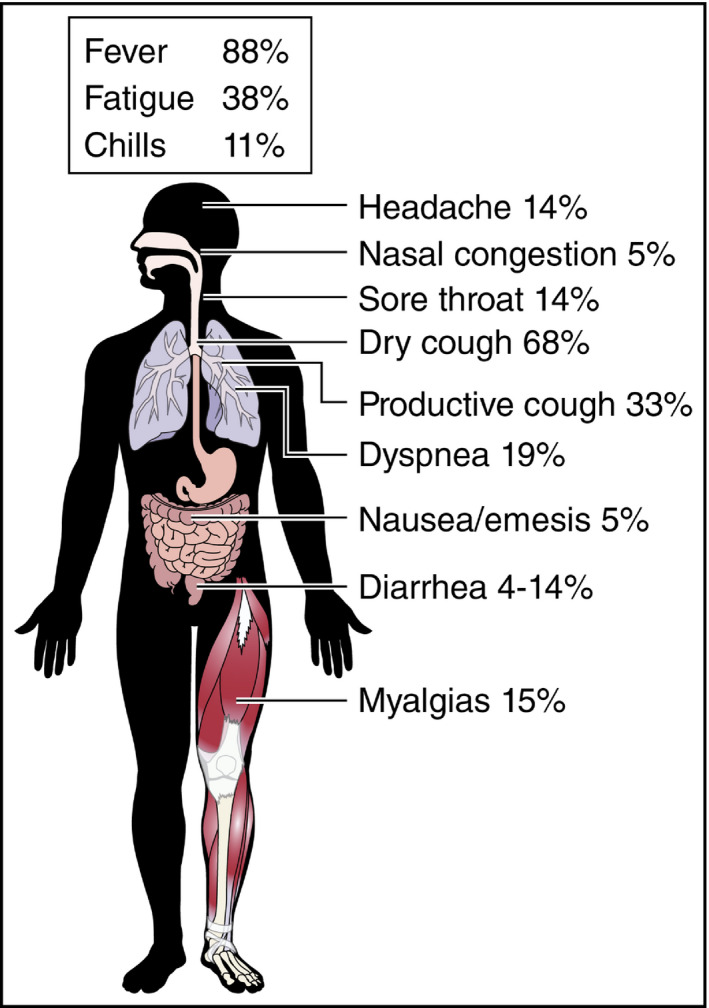
Wide range of symptoms in patients with COVID‐19 (reproduced, with permission, from Clerkin KJ et al, 2020) 22
Figure 2.
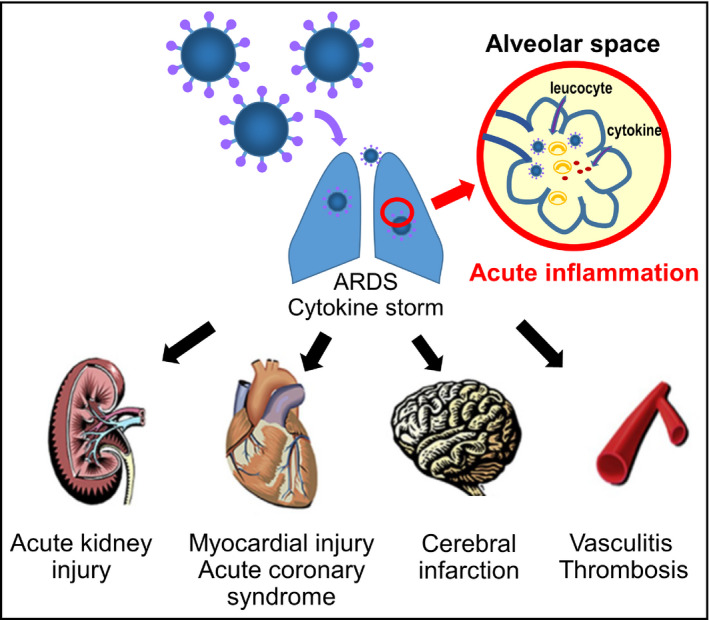
Variety of organ damage seen in patients with COVID‐19. ARDS, acute respiratory distress syndrome
Early clinical experience suggested that older age and the presence of a number of comorbidities, including hypertension, cardiovascular disease, diabetes mellitus and chronic respiratory disease increased the risk of death in patients with COVID‐19. 2 , 3 In addition, the renin‐angiotensin aldosterone (RAS) system (specifically the angiotensin‐converting enzyme 2 [ACE2] protein) has been identified as playing an important role in facilitating entry of coronaviruses, including SARS‐CoV‐2, into target cells, especially in the lungs. 4 , 5 Therefore, it has been suggested that angiotensin receptor blockers (ARBs) and ACE inhibitors, which affect ACE2 expression, may influence the susceptibility to and severity of infection with SARS‐CoV‐2. 6 , 7 , 8 , 9 , 10 , 11
Hypertension is very common, affecting an estimated 1.39 billion individuals worldwide, 12 and the prevalence of hypertension increases with age (affecting approximately 70% of older adults). 13 In addition, RAS inhibitors such as ACE inhibitors and ARBs are recommended and widely used for the treatment of hypertension. 14 , 15 , 16 However, hypertension is not a single clinical entity, but it instead manifests as a number of different phenotypes. In Asians, the disease is characterized by salt sensitivity, high rates of masked hypertension, exaggerated morning BP surge, and nocturnal hypertension. 17 Nearly half of all patients with hypertension worldwide (44%) live in south or east Asia. 18
The HOPE Asia Network was established in 2016 and is a member of the World Hypertension League. 19 , 20 The mission of the HOPE Asia Network is to improve the management of hypertension and organ protection toward achieving “zero” cardiovascular events in Asia. 19 , 20 This has become even more relevant in the current pandemic, with high rates of infection in several Asian countries.
This guidance from the HOPE Asia Network summarizes the latest findings on COVID‐19 and hypertension, including evidence‐based recommendations for the management of hypertension during the current pandemic.
2. HYPERTENSION AS A RISK FACTOR IN PATIENTS WITH COVID‐19
Clinical Question 1. Is hypertension a risk factor for COVID‐19?
Pre‐existing hypertension appears to be common in patients with severe COVID‐19. However, there is little direct evidence to indicate that hypertension itself is a risk factor for infection or aggravation of the disease independent of aging or other COVID‐19 risk factors.
On March 20, 2020, the Italian Institute of Health announced that there had been 3200 COVID‐19 deaths in Italy. 21 The patients who died had an average age of 78.5 years (median 80 years, range 31‐103 years) and 98.7% had at least one comorbidity. 21 Hypertension was a common comorbidity in Italian cases, affecting 73.8% of patients, 52% of whom were taking ARBs or ACE inhibitors. 21 However, there are number of factors that could potentially confound a possible relationship between hypertension and severe COVID‐19 (Table 1). 22 , 23 , 24 , 25 , 26 , 27 The first is age: both severe COVID‐19 and hypertension are common in the elderly. In addition, the identified risk factors (Table 1) are generally associated with aging and/or vascular disorders, both of which are common in patients with hypertension. Therefore, the risk of developing severe COVID‐19 is more likely to be due to underlying vascular endothelial dysfunction and/or organ damage than high blood pressure (BP) per se. ACE2 receptors are expressed by endothelial cells, 28 and post‐mortem examinations have detected the presence of viral infection in endothelial cells. 29
Table 1.
Risk factors for progression/severity of COVID‐19
| Aging |
| Hypertension |
| Diabetes mellitus |
| Smoking |
| Cardiovascular disease (heart failure, stroke, angina, myocardial infarction) |
| Chronic obstructive pulmonary disease |
| Chronic kidney disease |
| Malignancy (especially receiving current treatment with chemotherapy or radiotherapy) |
3. THERAPY WITH RENIN‐ANGIOTENSIN SYSTEM INHIBITORS
Clinical Question 2. Is it safe to continue treatment with ACE inhibitors or ARBs?
As of early May 2020, there is no clinical data showing that use of ACE inhibitors or ARBs increases the risk of infection with the SARS‐CoV‐2 virus or worsens the course of COVID‐19 disease. Scientific societies including the American Heart Association, European Society of Cardiology, Japanese Circulation Society, and Japanese Society of Hypertension recommend continuation of ACE inhibitor or ARB therapy in patients with hypertension.
3.1. Mechanisms linking COVID‐19 and ACE2
The spike protein on the surface of SARS‐CoV‐2 binds to the extracellular domain of transmembrane ACE2, with S protein priming by transmembrane serine protease 2 (TMPRSS2), to gain entry to host cells (Figure 3). 4 , 5 , 30 ACE2 plays a regulatory role in the RAS, converting angiotensin I (Ang I) into angiotensin 1‐9 (Ang 1‐9) or angiotensin II (Ang II) into angiotensin 1‐7 (Ang 1‐7). 31 , 32 Currently, available data reflect a possible role for ACE2 in heart failure, myocardial infarction, hypertension, and the cardiovascular complications of diabetes mellitus, and preclinical investigations suggest that activation of ACE2 might have the potential to protect against hypertension and cardiovascular disease. 31 , 33 , 34 , 35 In addition, angiotensin 1‐7 appears to counteract the negative effects of Ang II, attenuating inflammation, suppressing vascular permeability and having vasorelaxant effects. 36 , 37 , 38 Furthermore, ACE2 in the lungs and the renin‐angiotensin system has been shown to play a role in the pulmonary manifestations of coronavirus infection. 39
Figure 3.
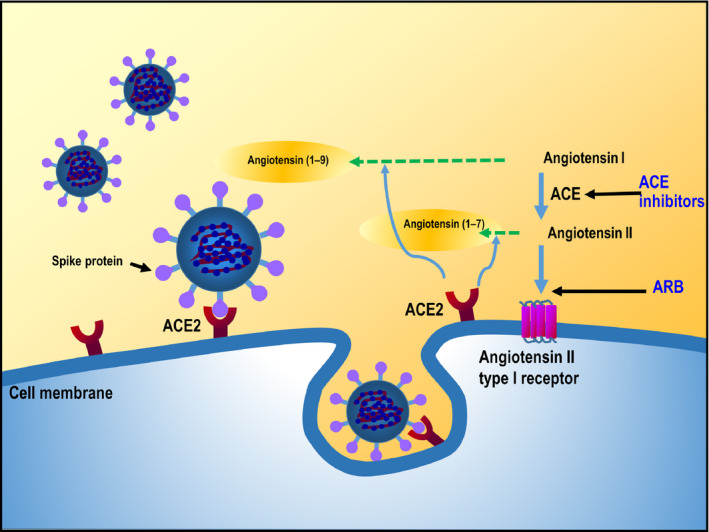
SARS‐CoV‐2 and the renin‐angiotensin system. ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker
Interaction of Ang II with angiotensin type 1 receptors (AT1R) activates A Disintegrin And Metalloproteinase 17 (ADAM17) on the cell membrane via phosphorylation. 40 In turn, ADAM17 cleaves the precursors of tumor necrosis factor‐α (TNFα) and interleukin (IL)‐6 receptor‐α (IL‐6Rα) in the cell membrane to release TNFα and soluble IL‐6Rα. 41 TNFα activates the nuclear transcription factor system NF‐κB to induce the production of various inflammatory cytokines, including IL‐6 (Figure 4). 42 This represents a potential mechanism for the cytokine storm seen in some patients with COVID‐19 and highlights the potential for agents blocking cytokine pathways (especially the IL‐6‐STAT3 axis) in managing COVID‐19‐related cytokine storm. 43
Figure 4.
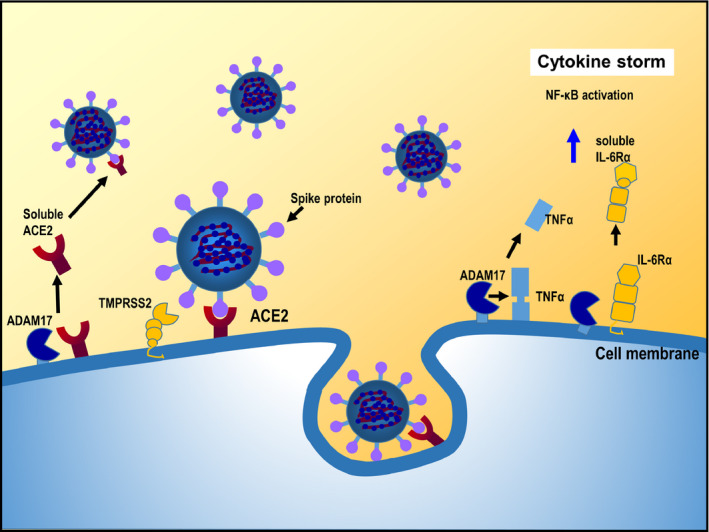
Cytokine storm associated with SARS‐CoV‐2 infection. ACE, angiotensin‐converting enzyme; ADAM17, A Disintegrin And Metalloproteinase 17; IL‐6Rα, interleukin‐6 receptor‐α; TMPRSS2, transmembrane serine protease 2; TNFα, tumor necrosis factor‐α
The fact that the SARS‐CoV‐2 virus uses ACE2 as a mechanism to enter and infect cells meant that there was concern that cells with high ACE2 expression would be most susceptible to infection with SARS‐CoV‐2. Given that ARB and ACE inhibitors have been shown experimentally to increase expression of ACE2 on cell membranes, 28 , 44 there was much discussion about the potential for higher infection rates and more severe disease in patients being treated with these agents.
3.2. Current clinical evidence
Despite the theoretical possibility that use of RAS inhibitors increases the risk of infection with SARS‐CoV‐2 and the severity of COVID‐19 illness, analyses including patients from the current pandemic indicate that this does not seem to be the case (Table 2).
Table 2.
Summary of studies investigating COVID‐19 in patients with hypertension, including those receiving renin‐angiotensin system inhibitors
| AuthorReference | Location | Design | Patients | Evidence |
|---|---|---|---|---|
| Li, et al 48 | Hubei province, China | Retrospective case series | 1178 pts hospitalized with COVID‐19 |
362 pts (31%) had HTN; 115/362 (32%) were taking ACEI/ARBs. In‐hospital mortality in pts with vs without HTN: 21% vs 11%. Use of ACEI/ARBs did not differ between pts with severe vs non‐severe COVID‐19 illness (33% vs 33%; P = .65), or between non‐survivors and survivors (27% vs 33%; P = .34). |
| Mancia, et al 45 | Lombardy region, Italy | Population‐based case‐control study | 6272 cases with confirmed COVID‐19 and 30,759 matched controls |
Use of ACEI/ARBs was more common in cases vs controls because cases had a higher rate of CVD. After adjustment for coexisting conditions, there was no association between use of ACEI or ARB and the risk of COVID‐19 infection (OR 0.96, 95% CI 0.87‐1.07 and OR 0.95, 95% CI 0.87‐1.07). |
| Mehta, et al 46 | Ohio & Florida, USA | Retrospective cohort study | 18,472 pts tested for COVID‐19 |
2285 pts were taking ACEIs or ARBs. There was no significant association between ACEI/ARB use and COVID‐19 test positivity (overlap propensity score‐weighted OR 0.97, 95% CI 0.81‐1.15). |
| Reynolds, et al 47 | New York, USA | Retrospective observational study | 12,594 pts tested for COVID‐19 |
4357 pts (35%) had a history of HTN; 2573/4357 (59.1%) had a positive test result; and 634 of these (25%) had severe illness. In propensity score‐matched groups, the likelihood of a positive test in pts with HTN was not affected by treatment with an ACEI or ARB. |
| Yang, et al 49 | Wuhan, China | Retrospective observational study | 126 pts with HTN and COVID‐19 and 125 age‐ and sex‐matched controls with COVID‐19 but no HTN |
Levels of hs‐CRP (P = .049) and procalcitonin (P = .008) were significantly lower in pts with HTN who were vs were not receiving ACEI/ARBs. The proportion of critical pts (9% vs 23%; P = .061) and the death rate (5% vs 13%; P = .216) were numerically but not significantly lower in the ACEI/ARB vs non‐ACEI/ARB group. |
| Zhang, et al 50 | Hubei province, China | Multicenter (9), retrospective observational study | 1128 pts with HTN and COVID‐19 |
188 pts were taking ACEI/ARB. In a Cox model adjusted for age, sex, comorbidities, and in‐hospital medication, all‐cause mortality was lower in the pts who were vs were not receiving ACEI/ARB (HR 0.37, 95% CI 0.150.89; P = .03). |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; hs‐CRP, hig sensitivity C‐reactive protein; HTN, hypertension; OR, odds ratio; pts, patients.
The effect of hypertension or therapy with ACE inhibitors or ARBs has been evaluated in at least three published studies to date (Table 2). 45 , 46 , 47 Reynolds et al looked at history of antihypertensive usage in 12 594 patients undergoing COVID‐19 testing in New York, USA. 47 They did not find any association between the use of ACE inhibitors, ARBs, beta‐blockers, calcium channel blockers or thiazide diuretics and the likelihood of a positive or negative result on COVID‐19 testing. 47 Also in the United States, Mehta and colleagues failed to find any significant association between the use of ACE inhibitors or ARBs and COVID‐19 test positivity. 46 Similar findings were reported in a population case‐control study from Italy. 45
Data from four studies published by early May 2020 also failed to find a significant association between RAS inhibitor use and worse outcomes in patients with COVID‐19 (Table 2). 48 , 49 , 50 In one retrospective case series, the proportion of patients using ACE inhibitors or ARBs did not differ significantly between those with severe vs non‐severe COVID‐19, or between survivors and non‐survivors. 48 However, the in‐hospital COVID‐19 mortality rate was higher in patients with vs without hypertension (21% vs 11%). 48 In the other studies, death rates for patients taking ACE inhibitors and/or ARBs were actually lower than those in patients not receiving these antihypertensive therapies. 49 , 50 One of the studies from China reported that levels of the inflammatory markers high sensitivity C‐reactive protein and procalcitonin were significantly lower in patients with hypertension who were vs were not receiving ACE inhibitors or ARBs. 49
4. BIOMARKERS OF COVID‐19‐RELATED COMPLICATIONS
Clinical Question 3. What are the biomarkers of severe COVID‐19?
In addition to ARDS, patients with severe infection can develop myocardial injury and cytokine storm, resulting in heart failure, arteriovenous thrombosis (venous thromboembolism, acute coronary syndrome, cerebral infarction), and acute kidney injury. Biomarkers for these complications are troponin, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), D‐dimer, and serum creatinine.
High levels of a number of biomarkers are indicative of severe COVID‐19 (Table 3). One of the most important biomarkers in patients with COVID‐19 is troponin, which indicates the presence of myocardial injury. D‐dimer and IL‐6 are also important. D‐dimer indicates the presence of arterial microthrombus and venous thrombosis (pulmonary embolism and deep vein thrombosis) and disseminated intravascular coagulation (DIC). IL‐6 is an inflammatory marker, suggesting the presence of cytokine storm, while NT‐proBNP and creatinine are biomarkers of heart failure and renal damage, respectively.
Table 3.
Biomarkers for progression of COVID‐19‐related complications
| Biomarker | Clinical condition |
|---|---|
| Oxygen saturation <94% | Acute respiratory distress syndrome |
| Troponin | Myocardial injury |
| D‐dimer | Thrombosis |
| Amino‐terminal pro‐B‐type natriuretic peptide | Heart failure |
| Creatinine | Kidney injury |
| C‐reactive protein | Cytokine storm |
| Interleukin‐6 | Cytokine storm |
4.1. Troponin and myocardial injury
There are two possible mechanisms of cardiovascular damage in COVID‐19. The first is direct viral infection of myocardial and vascular cells, and the other is a systemic inflammatory reaction (or cytokine storm) (Figure 5). Myocardial injury at the time of admission or due to disease progression is a strong indicator of poor prognosis in patients with COVID‐19. Troponin is a highly sensitive and well‐known marker of myocardial injury.
Figure 5.
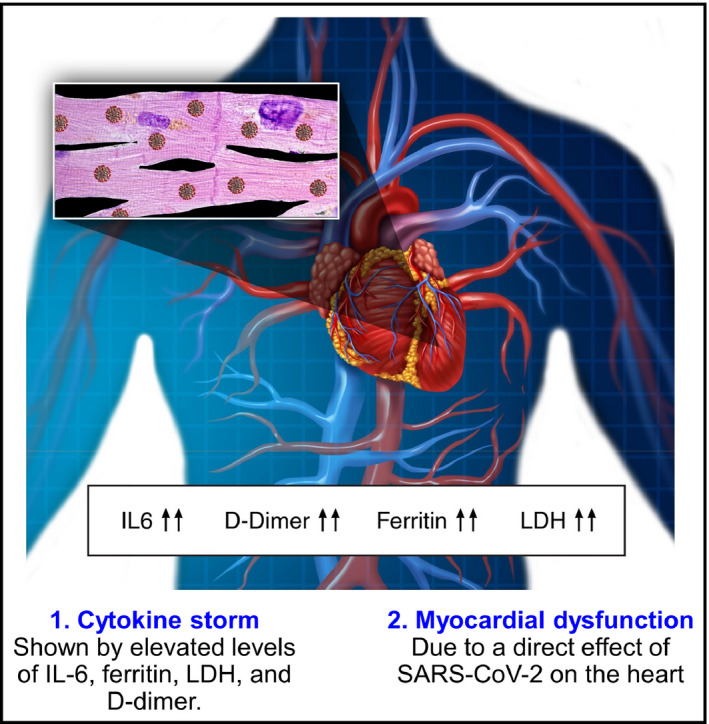
Mechanisms of myocardial injury in patients with COVID‐19 (reproduced, with permission, from Clerkin KJ et al, 2020) 22 IL6, interleukin‐6; LDH, lactate dehydrogenase
A systematic review and meta‐analysis of data published between 1 December 2019 and 27 March 2020 including 4189 patients from 28 studies showed a significant trend for higher levels of cardiac biomarkers in patients with more severe COVID‐19. 51 On meta‐regression analysis, the only factor significantly associated with higher levels of cardiac injury biomarkers was the presence of hypertension (P = .03). In addition, more severe COVID‐19 markedly increased the risk of acute cardiac injury (risk ratio vs mild disease 5.99, 95% confidence interval [CI] 3.04‐11.80; P < .001). In turn, the risk of COVID‐19‐related death was significantly increased in the presence of acute cardiac injury (risk ratio 3.85, 95% CI 2.13‐6.96). 51 Another analysis also reported that patients with severe COVID‐19 had significantly higher levels of troponin than those without severe disease (standardized mean difference 25.6 ng/L, 95% CI 6.8‐44.5 ng/L). 52
Early data from Wuhan, China, showed that 12% of patients admitted to hospital with COVID‐19 had acute cardiac injury. 53 Subsequent analyses investigated associations between underlying cardiovascular disease or myocardial injury and mortality in hospitalized patients with COVID‐19 (n = 187). 23 Patients with vs without myocardial injury (defined as elevated troponin levels) were significantly older (74 vs 60 years; P < .001) and more likely to have hypertension (59.8% vs 23.4%; P < .001). 23 , 25 During hospitalization, those with myocardial injury had higher rates of ARDS (58.5% vs 14.7%; P < .001), acute renal injury (8.5% vs 0.3%; P < .001) and death (51.2% vs 4.5%; P < .001). The appearance of myocardial injury increased the risk of death by 4.26‐fold compared to patients without myocardial damage, independent of cardiac function based on ECG and echocardiography findings (Figure 6). 23 , 25
Figure 6.
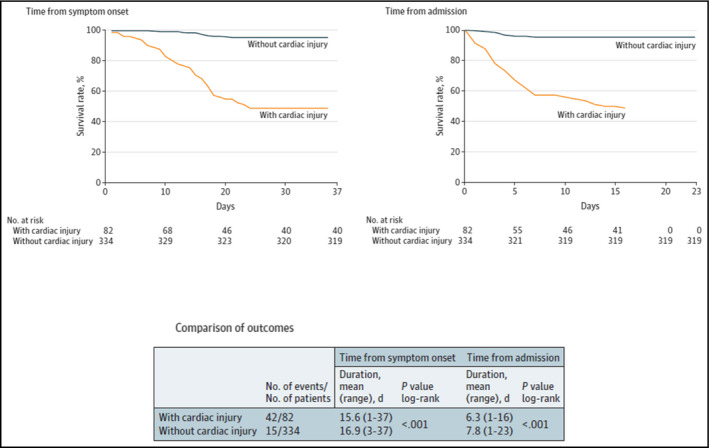
Prognosis in patients with COVID‐19, with or without cardiac injury (reproduced, with permission, from Shi et al, 2020) 25
4.2. D‐dimer
D‐dimer is a biomarker that reflects activation of coagulation and fibrinolysis. 54 D‐dimer levels of >2 µg/mL were an independent predictor of in‐hospital death in patients hospitalized with COVID‐19 in Wuhan, China (hazard ratio 51.5, 95% CI 12.9‐206.7; P < .001). 55 The 2 µg/mL cutoff had 92% sensitivity and 83% specificity for predicting in‐hospital mortality and therefore might be a useful biomarker for predicting outcome and informing treatment decisions in patients with COVID‐19. The cumulative rate of thromboembolic events in patients admitted to hospital in Milan, Italy with COVID‐19 (n = 388) was 21% (28% of those in intensive care and 7% for those not in intensive care) despite use of thromboprophylaxis in all patients admitted to the intensive care unit and three‐quarters of those treated on a general ward. 56 About half of all thromboembolic events occurred within 24 hours of hospitalization, and 2% of patients developed DIC. 56 A consensus statement on the prevention and treatment of venous thromboembolism‐associated COVID‐19 infection has been published. 57 This recommends that patients with COVID‐19 undergo assessment for the risk of venous thromboembolism and bleeding and are monitored regularly to facilitate the diagnosis and treatment of venous thromboembolism, and the use of strategies (pharmacologic and/or mechanical) to prevent venous thromboembolism. 57
5. CLINICAL PATIENT MANAGEMENT IN THE COVID‐19 ERA
Key points regarding the clinical management of COVID‐19, particularly in patients with hypertension, based on evidence published before May 5, 2020, are shown in Table 4.
Table 4.
Clinical practice guidance for patient management in the COVID‐19 era (based on evidence available up to May 5, 2020)
| COVID‐19 and Comorbidities: Assessment and Management |
|---|
|
|
|
|
|
|
|
|
|
Data that have been rapidly compiled during the pandemic to date indicate that there are a number of factors and biomarkers that can be used to identify patients with COVID‐19 who are at high risk of more severe disease and adverse outcomes, including death. Age seems to be the most important risk factor, especially for COVID‐19‐related death, and hypertension is the most common comorbidity in COVID‐19‐positive deceased patients. 21 Other comorbidities such as cardiovascular diseases, smoking, chronic lung disease, chronic kidney disease, and a suppressed immune system also increase the risks associated with COVID‐19 infection, especially when multiple comorbidities exist in the same patient. 21 Thorough history taking and baseline assessments are therefore important. Another important screening tool is oxygen saturation, which indicates severe disease if ≤94% on admission. 3 , 58 The relevance of ability to tolerate hypoxia was highlighted in an analysis from China showing that COVID‐19 patients of high‐altitude origin had a lower mortality risk than those from lower altitudes. 59 Determination of relevant biomarkers, especially troponin and D‐dimer provides an indication of myocardial injury and thrombotic risk, and can help inform appropriate treatment strategies.
For non‐hospitalized patients with COVID‐19, ongoing management of comorbid conditions is essential to minimize risk. This includes lifestyle factors such as diet and sleep, along with maintaining regular medications (eg, antihypertensives and antidiabetics). Patients with hypertension on current therapy with ACE inhibitors or ARBs can continue treatment without any negative effects on COVID‐19 outcomes. RAS inhibitors are particularly beneficial in patients who have heart failure or renal disease because they have a positive effect on prognosis. For older patients with hypertension and no other comorbidities and risk factors, calcium channel antagonists might be a good option for antihypertensive therapy. Maintaining good glycaemic control is also important because this might help reduce the risk of infection with SARS‐CoV‐2 and the severity of COVID‐19. 60 In addition, regular monitoring of home BP will help to ensure the achievement and maintenance of BP targets in patients with hypertension.
Lockdown requirements could impact on the ability of individuals to get regular exercise. Regular exercise is important for maintaining health status 61 and to counteract the negative consequences of cardiovascular, metabolic, and respiratory diseases. 62 Even if unable to get outside, continuing some form of home‐based exercise would be beneficial, especially in older adults. 63 Maintaining regular exercise could help to ensure that the current viral pandemic does not contribute to the worldwide obesity epidemic, which in turn increases the rate of cardiovascular and metabolic comorbidities. 64 Ensuring that the world population is not burdened by high rates of noncommunicable diseases might help lessen the impact of future pandemics given that patients with comorbidities tend to fare poorly during an infectious disease pandemic. 64 Another potential benefit of physical activity is its ability to stimulate immune function, 65 something that is highly relevant during a global pandemic.
Health care professionals should also be aware of the potential non‐infectious impacts of COVID‐19. For example, non‐infected individuals might be at risk of developing stress‐related physical health conditions (such as gastrointestinal disturbances and cardiovascular disease) and psychological disturbances (such as anxiety and depression).
6. TELEMEDICINE DURING COVID‐19
Strict lockdown and social distancing rules are being enforced in many countries to slow the spread of the novel coronavirus. In addition, a large proportion of “elective” or “non‐essential” procedures have been postponed or canceled to allow health care systems to cope with the influx of infectious disease cases. This has created a requirement for a large proportion of health consultations to be conducted remotely. Telemedicine strategies are ideally suited to facilitating patient management in the absence of face‐to‐face consultations, and the value of this approach (which has otherwise been slow to be widely used in clinical practice) has become clear. 66 , 67 One of the hidden “blessings” of the COVID‐19 pandemic may be the widespread adoption of telemedicine approaches to improve patient management.
Out‐of‐office BP monitoring is a recommended approach for the diagnosis and management of hypertension. 14 , 15 , 16 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 Therefore, this field of medicine is better placed than many to be able to continue to effectively manage patients during a global pandemic.
New information and communication technology‐based home BP monitoring devices that perform automatic, fixed‐interval BP measurement during sleep and store or transmit the data could facilitate a novel approach to patient management. 76 Validated wearable technologies for evaluation of home BP 77 , 78 might also be useful for patient monitoring and management during the COVID‐19 outbreak. Telemedicine‐based strategies for managing BP were implemented and used effectively during the aftermath of the Great East Japan earthquake and tsunami in March 2011, 79 highlighting their potential for use during the COVID‐19 pandemic to ensure that patients with hypertension have well‐controlled BP. This has the potential to help mitigate the negative effects of hypertension on prognosis in patients with COVID‐19.
7. CONCLUSIONS
Patients with hypertension are at increased risk of morbidity and mortality if they become infected with SARS‐CoV‐2, although this is confounded by other factors such as age and vascular disorders. However, all usual antihypertensive therapy including RAS inhibitors should continue. Physicians need to take a holistic approach to patient management due the wide range of possible complications, and biomarkers can provide important prognostic information. Overall, multidisciplinary management of COVID‐19 based on a rapidly growing body of evidence will help ensure the best possible outcomes for patients, including those with risk factors such as hypertension.
CONFLICT OF INTEREST
All authors report no potential conflicts of interest in relation to this article.
ACKNOWLEDGMENTS
We would like to express our gratitude to Ayako Okura, the academic editorial coordinator of the Department of Cardiology, Jichi Medical School, and Yukiko Suzuki, the academic coordinator, for their assistance with data gathering and figure preparation. Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by Jichi Medical University.
Kario K, Morisawa Y, Sukonthasarn A, et al; Hypertension Cardiovascular Outcome Prevention, Evidence in Asia (HOPE Asia) Network . COVID‐19 and hypertension–evidence and practical management: Guidance from the HOPE Asia Network. J Clin Hypertens. 2020;22:1109–1119. 10.1111/jch.13917
REFERENCES
- 1. Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020; [Epub ahead of print], 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020; [Epub ahead of print], 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; [Epub ahead of print], 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danser AHJ, Epstein M, Batlle D. Renin‐angiotensin system blockers and the COVID‐19 pandemic: at present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension. 2020;75(6):1382–1385. 10.1161/HYPERTENSIONAHA.1120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors‐lessons from available evidence and insights into COVID‐19. Hypertens Res. 2020;43(7):648–654. 10.1038/s41440-41020-40455-41448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020; [Epub ahead of print], 10.1093/cvr/cvaa1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuster GM, Pfister O, Burkard T, et al. SARS‐CoV2: should inhibitors of the renin‐angiotensin system be withdrawn in patients with COVID‐19? Eur Heart J. 2020; [Epub ahead of print], 10.1093/eurheartj/ehaa1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugimoto T, Mizuno A, Kishi T, et al. Coronavirus disease 2019 (COVID‐19) information for cardiologists ‐ systematic literature review and additional analysis. Circ J. 2020; [Epub ahead of print], 10.1253/circj.CJ-1220-0302 [DOI] [PubMed] [Google Scholar]
- 11. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382:1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:329‐322. [DOI] [PubMed] [Google Scholar]
- 14. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 15. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882‐1888. [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 17. Kario K, Park S, Chia YC, et al. 2020 Consensus summary on the management of hypertension in Asia from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020;22:351‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NCD Ris k Factor Collaboration . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kario K. The HOPE Asia Network activity for "zero" cardiovascular events in Asia: overview 2020. J Clin Hypertens (Greenwich). 2020;22:321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kario K, Chia YC, Sukonthasarn A, et al. Diversity of and initiatives for hypertension management in Asia‐Why we need the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020;22:331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Istituto Superiore Di Santia . Characteristics of COVID‐19 patients dying in Italy. Report based on data available on March 20th, 2020. Available from: https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf. Accessed 12 May 2020.
- 22. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation. 2020;141(20):1648–1655. 10.1161/CIRCULATIONAHA.1120.046941 [DOI] [PubMed] [Google Scholar]
- 23. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; [Epub ahead of print], 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020; [Epub ahead of print], 10.1093/cvr/cvaa1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020; [Epub ahead of print], 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605‐2610. [DOI] [PubMed] [Google Scholar]
- 29. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281‐292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1–7 axis of the Renin‐Angiotensin system in heart failure. Circ Res. 2016;118:1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double‐edged sword. Circulation. 2020; [Epub ahead of print], 10.1161/CIRCULATIONAHA.1120.047049. [DOI] [PubMed] [Google Scholar]
- 33. Shenoy V, Kwon KC, Rathinasabapathy A, et al. Oral delivery of Angiotensin‐converting enzyme 2 and Angiotensin‐(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69:805‐819. [DOI] [PubMed] [Google Scholar]
- 35. Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74:1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villalobos LA, San Hipolito‐Luengo A, Ramos‐Gonzalez M, et al. The angiotensin‐(1–7)/mas axis counteracts angiotensin II‐dependent and ‐independent pro‐inflammatory signaling in human vascular smooth muscle cells. Front Pharmacol. 2016;7:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrario CM. New physiological concepts of the renin‐angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferreira AJ, Santos RA, Bradford CN, et al. Therapeutic implications of the vasoprotective axis of the renin‐angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawai T, Forrester SJ, O'Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125:4‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scheller J, Chalaris A, Garbers C, Rose‐John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380‐387. [DOI] [PubMed] [Google Scholar]
- 42. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF‐kappa B. Annu Rev Cell Biol. 1994;10:405‐455. [DOI] [PubMed] [Google Scholar]
- 43. Hirano T, Murakami M. COVID‐19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(2):731‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin‐converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970‐976. [DOI] [PubMed] [Google Scholar]
- 45. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; [Epub ahead of print], 10.1001/jamacardio.2020.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020; [Epub ahead of print]. 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang G, Tan Z, Zhou L, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID‐19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76(1):51–58. 10.1161/HYPERTENSIONAHA.1120.15143 [DOI] [PubMed] [Google Scholar]
- 50. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671–1681. 10.1161/CIRCRESAHA.1120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: a systemic review and meta‐analysis. Prog Cardiovasc Dis. 2020; [Epub ahead of print], 10.1016/j.pcad.2020.1004.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Long Y, Xiao H, Yang J, Toulon P, Zhang Z. Use of D‐dimer in oral anticoagulation therapy. Int J Lab Hematol. 2018;40:503‐507. [DOI] [PubMed] [Google Scholar]
- 55. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(06):937‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID‐19) – China, 2020. China CDC Weekly. 2020;2:133‐142. [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou K, Yang S, Jia P. Towards precision management of cardiovascular patients with COVID‐19 to reduce mortality. Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID‐19 epidemic. Diabetes Metab Syndr. 2020;14:211‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124:799‐815. [DOI] [PubMed] [Google Scholar]
- 62. Ozemek C, Lavie CJ, Rognmo O. Global physical activity levels ‐ need for intervention. Prog Cardiovasc Dis. 2019;62:102‐107. [DOI] [PubMed] [Google Scholar]
- 63. Jimenez‐Pavon D, Carbonell‐Baeza A, Lavie CJ. Physical exercise as therapy to fight against the mental and physical consequences of COVID‐19 quarantine: Special focus in older people. Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hall G, Laddu DR, Phillips SA, Lavie CJ, Arena R. A tale of two pandemics: how will COVID‐19 and global trends in physical inactivity and sedentary behavior affect one another? Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Laddu DR, Lavie CJ, Phillips SA, Arena R. Physical activity for immunity protection: inoculating populations with healthy living medicine in preparation for the next pandemic. Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bashshur R, Doarn CR, Frenk JM, Kvedar JC, Woolliscroft JO. Telemedicine and the COVID‐19 pandemic, lessons for the future. Telemed J E Health. 2020;26:571‐573. [DOI] [PubMed] [Google Scholar]
- 67. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID‐19 outbreak response systems. Am J Manag Care. 2020;26:147‐148. [DOI] [PubMed] [Google Scholar]
- 68. Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1‐47. [DOI] [PubMed] [Google Scholar]
- 69. Kario K, Shin J, Chen CH, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens (Greenwich). 2019;21:1250‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. National Institute for Health and Care Excellence . Hypertension: clinical management of primary hypertension in adults (update). Clinical guideline 127 (2011). Available from: https://www.nice.org.uk/guidance/cg127/chapter/1-guidance. Last accessed: Dec 2016.
- 71. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506‐525. [DOI] [PubMed] [Google Scholar]
- 72. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 73. Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 74. Park S, Buranakitjaroen P, Chen CH, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32:249‐258. [DOI] [PubMed] [Google Scholar]
- 75. Sharman JE, Howes FS, Head GA, et al. Home blood pressure monitoring: Australian expert consensus statement. J Hypertens. 2015;33:1721‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71:997‐1009. [DOI] [PubMed] [Google Scholar]
- 77. Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of a wrist‐type home nocturnal blood pressure monitor in the sitting and supine position according to the ANSI/AAMI/ISO81060‐2:2013 guidelines: Omron HEM‐9600T. J Clin Hypertens (Greenwich). 2019;21:463‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch‐type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060‐2:2013 guidelines: Omron HEM‐6410T‐ZM and HEM‐6410T‐ZL. J Clin Hypertens (Greenwich). 2019;21:853‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nishizawa M, Hoshide S, Okawara Y, Matsuo T, Kario K. Strict blood pressure control achieved using an ICT‐based home blood pressure monitoring system in a catastrophically damaged area after a disaster. J Clin Hypertens (Greenwich). 2017;19:26‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]


