Abstract
BACKGROUND
The lack of effective treatments against the 2019 coronavirus disease (COVID‐19) has led to the exploratory use of convalescent plasma for treating COVID‐19. Case reports and case series have shown encouraging results. This study investigated SARS‐CoV‐2 antibodies and epidemiological characteristics in convalescent plasma donors, to identify criteria for donor selection.
METHODS
Recovered COVID‐19 patients, aged 18‐55 years, who had experienced no symptoms for more than 2 weeks, were recruited. Donor characteristics such as disease presentations were collected and SARS‐CoV‐2 N‐specific IgM, IgG, and S‐RBD‐specific IgG levels were measured by enzyme‐linked immunosorbent assay (ELISA).
RESULTS
Whereas levels of N‐specific IgM antibody declined after recovery, S‐RBD‐specific and N‐specific IgG antibodies increased after 4 weeks from the onset of symptoms, with no significant correlation to age, sex, or ABO blood type. Donors with the disease presentation of fever exceeding 38.5°C or lasting longer than 3 days exhibited higher levels of S‐RBD‐specific IgG antibodies at the time of donation. Of the 49 convalescent plasma donors, 90% had an S‐RBD‐specific IgG titer of ≥1:160 and 78% had a titer of ≥1:640 at the time of plasma donation. Of the 30 convalescent plasma donors, who had donated plasma later than 28 days after the onset of symptoms and had a disease presentation of fever lasting longer than 3 days or a body temperature exceeding 38.5°C, 100% had an S‐RBD‐specific IgG titer of ≥1:160 and 93% had a titer of ≥1:640.
CONCLUSION
This study indicates that the S‐RBD‐specific IgG antibody reaches higher levels after 4 weeks from the onset of COVID‐19 symptoms. We recommend the following selection criteria for optimal donation of COVID‐19 convalescent plasma: 28 days after the onset of symptoms and with a disease presentation of fever lasting longer than 3 days or a body temperature exceeding 38.5°C. Selection based on these criteria can ensure a high likelihood of achieving sufficiently high S‐RBD‐specific IgG titers.
A novel human coronavirus, identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), has rapidly spread across the world, resulting in an international COVID‐19 pandemic. The World Health Organization (WHO) has announced that the outbreak of the novel coronavirus constitutes a global public health emergency. The full spectrum of COVID‐19 symptoms ranges from mild, self‐limiting respiratory tract illnesses to severe progressive pneumonia, multi‐organ failure, and death. 1 Unfortunately, no specific therapeutic agents or vaccines for COVID‐19 are available, apart from Lopinavir‐Ritonavir, which may be a promising treatment. 2 Several other therapies such as remdesivir and favipiravir are under investigation, but the antiviral effects of these drugs are not fully known. 3 , 4
Passive immunization for the treatment of human infectious diseases can be traced back to the past century. 5 Convalescent plasma has been tried to fight pathogens including Severe Acute Respiratory Syndrome Coronavirus (SARS‐CoV), 6 Ebola virus, 7 Middle East Respiratory Syndrome Coronavirus (MERS‐CoV), 8 and avian influenza A (H5N1) virus. 9 Few studies, however, have been conducted on the selection of donors to ensure therapeutic potency in convalescent plasma. 10 In 2007, the WHO Blood Regulators Network (BRN) issued a guidance document on the selection of donors in a pandemic. 11 The recent COVID‐19 outbreak worldwide has prompted the exploratory use of convalescent plasma in treating COVID‐19; and case reports and case series have shown encouraging results. 12 , 13
SARS‐CoV‐2 is an enveloped virus with four structural proteins: spike (S) protein, membrane (M) protein, enveloped (E) protein, and nucleocapsid (N) protein. 14 The S protein receptor‐binding domain (RBD) has been identified as a key target for therapeutic antibodies, as it plays essential roles in tropism and virus entry into host cells and can induce neutralizing antibodies and protective immunity. 15 , 16 , 17 , 18
To collect high‐quality convalescent plasma, potential donors with appropriate characteristics should donate plasma at the proper time. The selection of convalescent plasma donors and the timing of their donations are important to ensure therapeutic potency. In this study, we collected demographic information and health history from COVID‐19 convalescent plasma donors and investigated relevant viral serology, to identify the appropriate selection criteria for convalescent plasma donors.
METHODS
This study was conducted at the Blood Center of Wuhan, China, from February 12, 2020, to March 2, 2020. All convalescent plasma donors provided written informed consent. The study was approved by the Ethics Review Committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences.
Donor recruitment and sample collection
Patients who had recovered from COVID‐19 were recruited as blood donors for this study. All patients aged 18‐55 who had a confirmed COVID‐19 diagnosis in Wuhan, China as of February 12, 2020 were identified by searching hospital records. Prospective study subjects were contacted by phone to assess interest and study eligibility. Study eligibility was determined using a COVID‐19‐specific health history questionnaire based on the following criteria: 1) aged 18‐55 years; 2) eligible for blood donation; 3) diagnosed with COVID‐19; 19 4) had two subsequent negative COVID‐19 nasopharyngeal swab tests based on PCR (at least 24 hours apart); 5) had been discharged from the hospital for more than 2 weeks; and 6) had no COVID‐19 symptoms prior to convalescent plasma donation.
Demographic information on the donors was collected, including sex, age, initial symptoms, duration of the disease, presence and duration of fever, and ABO blood type.
Donor testing samples were collected on the day(s) of intended convalescent plasma donation, stored at 4°C, and processed within 4 hours.
Routine donor testing
We tested for infectious pathogens according to the Chinese national quality standards for blood establishment technical procedures (2019 edition). 20 We conducted ELISA testing for anti‐HIV1/2, anti‐HCV, syphilis antibody, and HBs Ag; we performed NAT testing for HIV, HBV, and HCV.
Testing for SARS‐CoV‐2 nucleic acid
All donor samples were tested for the SARS‐CoV‐2 RNA by quantitative RT‐PCR using the PerkinElmer New Coronavirus Nucleic Acid Detection Kit (PerkinElmer Healthcare Diagnostics Co., Ltd.). The assays were performed according to the manufacturer's instructions.
Detection of N‐specific IgM and IgG antibodies
N‐specific IgM and IgG antibodies were tested for using the enzyme‐linked immunosorbent assay (ELISA) (Livzon Pharmaceutical Group) according to the manufacturers’ instructions.
Detection of S‐RBD‐specific IgG antibody
The S‐RBD‐specific IgG ELISA assay was developed in‐house. In brief, we coated 96 well plates (Thermo Scientific) with 100 ng of recombinant RBD polypeptides (Sino Biological) per well. After overnight coating, the coating solution was decanted from the plates and non‐specific activity was blocked with PBS containing 1% skim milk at 37°C for an hour. Plasma samples were diluted 160, 320, 640, and 1280 folds with 0.5% Triton X‐100 phosphate‐buffered saline and 5% fetal calf serum (Gibco) and added to the plates. After washing, mouse‐to‐human secondary antibodies were added to the plates; we observed the plates for the horseradish peroxidase reaction. The OD values were calculated by measuring the change in the absorbance at 450 nm and 630 nm using an automatic microplate reader (Sunrise, Tecan GmbH). Results were reported as the S/CO value, calculated as the ratio of the OD value to the cutoff value. Titers were reported as the highest dilution when the ELISA assay was still positive and ranged between 1:160 and 1:1280 (ELISA endpoint dilution titers).
Correlation of the S‐RBD‐specific IgG ELISA and SARS‐CoV‐2 viral neutralization assay
We isolated SARS‐CoV‐2 virus from a COVID‐19 patient. The virus was cultured on Vero cells (American Tissue Culture Collection [ATCC], CCL‐81) and the viral neutralization activity was determined based on TCID50 (Median Tissue Culture Infectious Dose). 21 In brief, we incubated study subjects' sera at 56°C for 30 minutes to deactivate the complements and then diluted serially from 1:10, 1:20, 1:40, 1:80, to 1:160. The diluted serum was mixed with equal volumes of SARS‐CoV‐2 virus at a dose of 100 TCID50, then incubated at 37°C for one hour. The mixture was added in quadruplicates to Vero cells cultured in 96‐well microtiter plates, then removed after 1 hour and replaced with 200 mL fresh growth medium. Serum samples from healthy volunteers were used as the control. The cytopathic effect was observed 5 days after incubation. The neutralizing antibody titers were calculated using the Reed‐Muench method. 21
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc.). Pearson's r or Spearman rank‐order correlation was used for correlations. Nonlinear regression (curve fit) analysis was performed on the changes of antibody levels over time. One‐way analysis of variance followed by the Newman–Keuls test was used for comparison of all pairs. A p value of less than 0.05 was considered statistically significant.
RESULTS
Donor characteristics
In this study, 49 convalescent plasma donors were recruited successfully, including 33 males and 16 females. The median age was 37 years (IQR, 25‐54 years). The most common self‐reported symptoms at the onset of illness were fever (39, 80%) and cough (30, 61%). Other symptoms included shortness of breath, muscle aches, and diarrhea. The majority (43, 88%) of patients presented with more than one symptom, 27 (55%) had a fever with cough, 16 (33%) had a fever with muscle aches, and 10 (20%) had a fever and shortness of breath (Table 1). The average time from the onset of symptoms to plasma donation was 37.8 days (IQR, 23‐58 days). All plasma samples were negative for SARS‐CoV‐2 nucleic acid and the transfusion transmitted pathogens tested.
TABLE 1.
Demographics, baseline characteristics and clinical symptoms of 49 donors
| Donors (n = 49) | |
|---|---|
| Age, years | |
| Mean ± SD | 37 ± 7.7 |
| Range | 25‐54 |
| ≤29 | 6 (12%) |
| 30‐39 | 27 (55%) |
| 40‐49 | 11 (23%) |
| ≥50 | 5 (10%) |
| Sex | |
| Male | 33 (67%) |
| Female | 16 (33%) |
| Severity of disease* | |
| Mild | 24 (49%) |
| Moderate | 25 (51%) |
| Severe | 0 (0%) |
| Signs and symptoms | |
| Fever | 39 (80%) |
| Cough | 30 (61%) |
| Shortness of breath | 10 (20%) |
| Muscle ache | 19 (39%) |
| Diarrhea | 13 (27%) |
| More than one sign or symptom | 43 (88%) |
| Fever and cough | 27 (49%) |
| Fever and shortness of breath | 10 (20%) |
| Fever and muscle ache | 16 (33%) |
| Time of disease onset to donation, days | |
| Mean ± SD | 37.8 ± 8.3 |
| Range | 23‐58 |
| <28 | 5 (10%) |
| 28‐34 | 14 (28%) |
| 35‐41 | 15 (31%) |
| ≥42 | 15 (31%) |
The definition of disease classification was based on the Chinese diagnosis and treatment guidelines for the new coronavirus pneumonia (trial version 5). 19
The trend of changes over recovery time
Changes in N‐specific IgM levels showed a decreasing trend and had a negative correlation with time from the onset of symptoms to the plasma donation (r = −0.3591, p = 0.0056) (Fig. 1A). Changes in N‐specific IgG‐levels showed an upward trend and had a positive correlation with time from the onset of symptoms to the plasma donation (r = 0.2635, p = 0.0352) (Fig. 1B). S‐RBD‐specific IgG showed similar results (r = 0.4540, p = 0.0011) (Fig. 1C). The heat map of these antibodies over time also showed that the levels of S‐RBD‐specific IgG antibody in donors gradually increased, while N‐specific IgM antibody gradually decreased and remained at a lower level (Fig. 1E). To further confirm the trend of S‐RBD‐specific IgG antibody changes over time, we analyzed antibody levels according to different recovery times. The levels of S‐RBD‐specific IgG antibodies were lower in donors who donated within 28 days from the onset of symptoms than in those who donated later than 28 days from the onset of symptoms. The difference was statistically significant, with a p value of 0.012 (Fig. 1D).
Fig. 1.
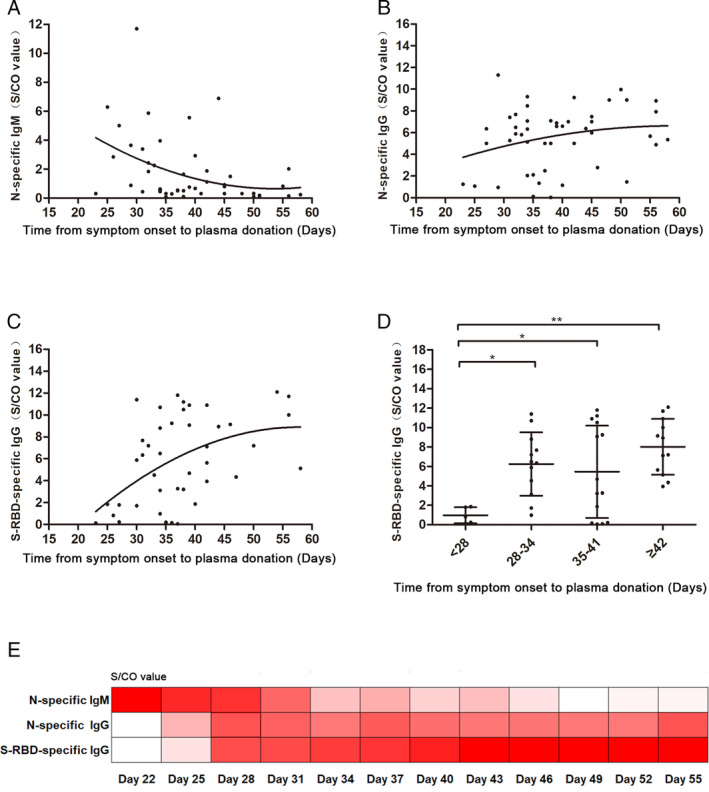
Nonlinear regression (curve fit) of SARS‐CoV‐2 antibodies levels. (A) The correlation between the time from disease onset to plasma donation and N‐specific IgM antibody level. There is an inverse correlation (r = −0.3591, p = 0.0056). (B) The correlation between the time from disease onset to plasma donation and N‐specific IgG antibody level. There is a positive correlation (r = 0.2635, p = 0.0352). (C) The correlation between the time from disease onset to plasma donation and S‐RBD‐specific IgG antibody level. There is a positive correlation (r = 0.4540, p = 0.0011). (D) The S‐RBD‐specific IgG antibody level at less than 28 days, 28‐34 days, 35‐41 days, and more than 42 days after disease onset. (E) The level of N‐specific IgM, IgG, and S‐RBD‐specific IgG antibodies during recovery time. The values are presented as the mean ± SD. *p < 0.05, **p < 0.01; NS, nonsignificant. [Color figure can be viewed at wileyonlinelibrary.com]
Correlation of S‐RBD‐specific IgG antibody and SARS‐CoV‐2 viral neutralization assay
We performed a correlation study between the SARS‐CoV‐2 viral neutralization titer and the S‐RBD‐specific IgG antibody titer. The results indicated a positive correlation (r = 0.6222, p = 0.0308) between the two assays. A serum neutralization titer of 1:80 is approximately equivalent to a titer of 1:1280 for S‐RBD‐specific IgG.
Correlation of donor characteristics and S‐RBD‐specific IgG antibody
A recent study has demonstrated that SARS‐CoV‐2 viral neutralization activity is correlated with the S‐RBD‐specific IgG antibody. 22 We assessed the correlation of the level of S‐RBD‐specific IgG antibody to donor factors including age, sex, initial symptoms, body temperature and duration of fever, and ABO blood type. The results showed that donors with high fevers exceeding 38.5°C or with fever lasting longer than 3 days had a high level of S‐RBD‐specific IgG (Fig. 2A, B). Notable, changes in antibody levels were not correlated to age, sex, or blood type (Note: we collected only one case of type AB) (Fig. 2C, D, and E).
Fig. 2.
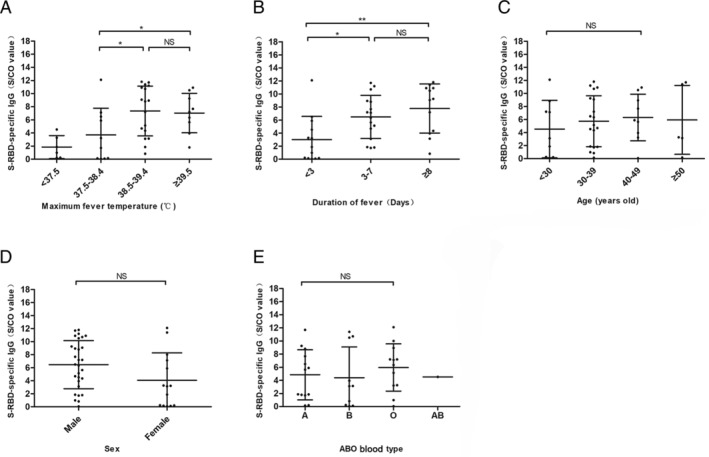
Correlation of donor characteristics and S‐RBD‐specific IgG antibody. (A) The S‐RBD‐specific IgG antibody level in non‐febrile (<37.5°C), mild‐febrile (37.5‐38.4°C), moderate‐febrile (38.5‐39.4°C), and extreme‐febrile (≥39.5°C) donors. (B) The S‐RBD‐specific IgG antibody level in donors with fevers lasting <3 days, 3‐7 days, and ≥8 days. (C) The S‐RBD‐specific IgG antibody level of donors <30 years old, 30‐39 years old, 40‐49 years old, and ≥50 years old. (D) The S‐RBD‐specific IgG antibody level in male and female donors. (E) The S‐RBD‐specific IgG antibody level of A, B, O, and AB blood type donors. The differences between the groups were analyzed using the Newman–Keuls test and values are presented as the mean ± SD. *p < 0.05, **p < 0.01; NS, nonsignificant.
Optimal selection of COVID‐19 convalescent plasma donors
To obtain optimal convalescent plasma donor selection criteria, we analyzed the titer distribution of the S‐RBD‐specific IgG antibody in convalescent plasma donors under different selection conditions. Among the 49 convalescent plasma donors, 90% had a titer of ≥1:160 and 78% had a titer of ≥1:640. Based on the selection criterion of donating plasma 4 weeks after the onset of symptoms, there were 42 convalescent plasma donors, of whom 90% had a titer of ≥1:160 and 84% had a titer of ≥1:640. Considering the additional selection criterion of a fever lasting longer than 3 days or a body temperature exceeding 38.5°C, there were 30 convalescent plasma donors, of whom 100% had a titer of ≥1:160 and 93% had a titer of ≥1:640 (Fig. 3). Of these, 29 donors had fever lasting longer than 3 days, and 27 donors had body temperatures exceeding 38.5°C during the fever (Fig. 3). Figure 4 shows the antibody titer distribution before and after the selection.
Fig. 3.
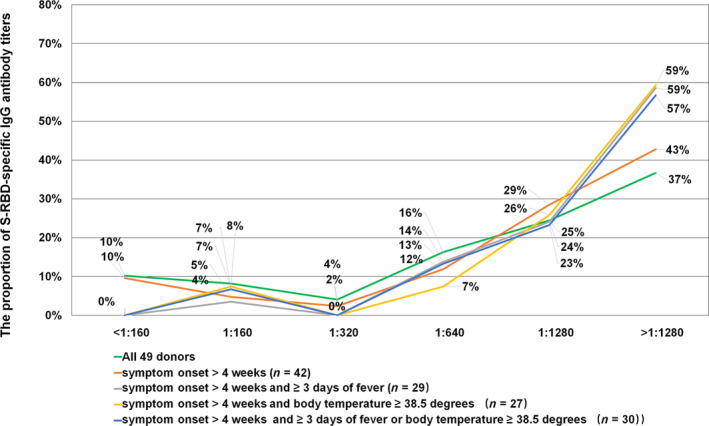
S‐RBD‐specific IgG antibody titers based on different selection criteria. The green line represents a total of 49 donors. The red line represents the distribution of antibody titer in donors with more than 4 weeks after symptom onset. The gray line represents the titer in donors with a fever lasting longer than 3 days at disease presentation and more than 4 weeks after symptom onset. The yellow line represents antibody titer in donors with body temperature exceeding 38.5°C at disease presentation and more than 4 weeks after symptom onset. The blue line represents antibody titer in donors with a fever lasting longer than 3 days or with body temperature exceeding 38.5°C at disease presentation and more than 4 weeks after symptom onset. [Color figure can be viewed at wileyonlinelibrary.com]
Fig. 4.
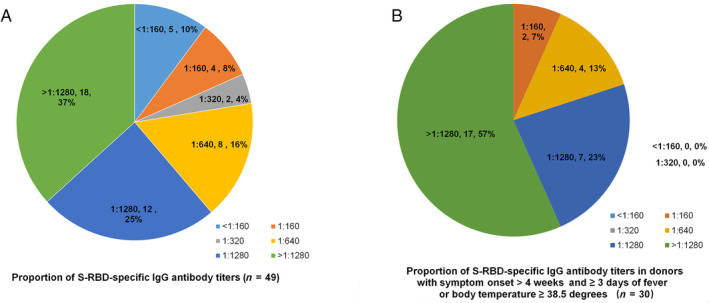
The proportion of S‐RBD‐specific IgG antibody titers. (A) The proportion of S‐RBD‐specific IgG antibody titers in 49 donors. (B) The proportion of S‐RBD‐specific IgG antibody titers in 30 donors with a fever lasting longer than 3 days or body temperatures exceeding 38.5°C at disease presentation and with more than 4 weeks after symptoms. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
The COVID‐19 pandemic has become a major public health challenge around the world. The use of convalescent plasma may be an effective and safe treatment option to help control the COVID‐19 pandemic. 12 , 23 There are now thousands of recovered COVID‐19 patients who could donate convalescent plasma. Establishing optimal selection criteria for COVID‐19 convalescent plasma donors is necessary to ensure convalescent plasma therapeutic potency and to optimize collection efficiency.
Here we have proposed a donor selection strategy for COVID‐19 convalescent plasma collection. A total of 49 convalescent plasma donors were analyzed, all of whom had mild or moderate COVID‐19 symptoms. At least 14 days after symptom resolution, we collected the convalescent plasma and analyzed SARS‐COV‐2 antibodies.
This study compares the changes of N‐specific IgM, IgG, and S‐RBD‐specific IgG antibody levels over time. Our data show that N‐specific IgM antibody levels continued to decline after 3 weeks of SARS‐COV‐2 infection and reached low levels after 6 weeks. At the same time, S‐RBD‐specific and N‐specific IgG antibodies exhibited an upward trend and continued to rise after 4 weeks from the onset of symptoms, which was consistent with the study by Ling et al. 24 Interestingly, the trends in increase showed by S‐RBD‐specific and N‐specific IgG antibodies were not quite the same; the former were more dramatic while the latter were milder. A recent study by Walls et al found that SARS‐CoV S‐murine polyclonal antibodies potently inhibit SARS‐CoV S mediated entry into cells and induce the production of protective neutralizing antibodies targeting S epitopes. 25 The RBD of Middle East respiratory syndrome coronavirus (MERS‐CoV), as a vaccine antigen, has been shown to induce neutralizing antibodies and protect mice transduced with a viral vector. 26 Thus, we predict that S‐RBD‐specific IgG may be a protective antibody and that its level would significantly increase in recovered patients. Cao et al. studied SARS‐CoV neutralizing antibody titers in 56 patients who recovered from SARS‐CoV infections. 27 Their findings show that SARS‐CoV IgG and neutralizing antibodies peaked at 4 months and then declined, reaching undetectable levels in 25.6% (IgG) and 16.1% (neutralizing antibodies) of study subjects at 36 months. Our observed positive correlation between S‐RBD‐specific IgG titer and viral neutralization titer is assuring. Ideally, COVID‐19 convalescent plasma products should be measured with viral neutralization antibody titer to ensure product potency. However, viral neutralization assay is time‐consuming to perform and requires the use of Level 3 bio‐safety cabinets, thus limiting its use in routine practice as a quality measurement for convalescent plasma. Using S‐RBD‐specific IgG titer as a surrogate marker for viral neutralization titer is far more practical and brings convalescent plasma product quality control for potency a step closer to reality.
Moreover, we observed a correlation between donor characteristics and S‐RBD‐specific IgG antibody. We found that donors who experienced disease symptoms including a body temperature exceeding 38.5°C or a fever lasting longer than 3 days had higher levels of S‐RBD‐specific IgG antibody titers in convalescent plasma. Although a recent study indicates that people with blood group A have a significantly higher risk of acquiring COVID‐19, whereas blood group O has a significantly lower risk for the infection, 28 our study did not show a significant correlation between S‐RBD‐specific IgG antibody and age, sex, or donor blood type.
Based on the results of this study, optimal COVID‐19 convalescent plasma donors with high S‐RBD‐specific IgG antibody levels (and most likely higher virus neutralization activity) can be selected based on the following criteria: disease symptoms comprising a fever lasting longer than 3 days or a body temperature exceeding 38.5°C. Providing additional selection criterion of donating plasma 4 weeks after the onset of symptoms could help achieve even higher S‐RBD‐specific IgG antibody levels.
Key elements to ensure an adequate supply of high‐quality COVID‐19 convalescent plasma products include the following: donor recruitment and selection, convalescent plasma collection, and product evaluation and quality control for the presence of adequate protective antibodies and the absence of transfusion transmitted pathogens. More studies, guidance, and regulations are needed to refine operation and product requirements for COVID‐19 convalescent plasma. This study also suggests that the antibody titers from asymptomatic individuals who are incidentally found to be positive for IgG antibodies to SARS‐CoV‐2 may be lower than those from symptomatic individuals. Future studies should evaluate this possibility.
Limitations
This study has several limitations. First, the sample size was small, so our selection criteria should be verified by larger studies. Second, the study design precludes a definitive conclusion regarding the potential therapeutic efficacy and requires clinical validation through clinical trials.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ling Li, Hanwei Chen, Qilu Lu, Ru Yang, Lei Zhao, Jue Wang, Haixia Xu, Chuanqiao Liu, Guoan Chen, Sitian Chen, Chenyue Li, and Jiajia Qiao were responsible for donor recruitment, plasma collection and performing all assays; Xunliang Tong and Rui He were responsible for data analysis and manuscript‐writing; Juntao Yang, Yanyun Wu, and Zhong Liu contributed to study design, research analysis, and manuscript revision.
ACKNOWLEDGMENTS
We would like to thank the following individuals for their guidance, expertise, and assistance with the study design: Peter W. Marks, MD PhD, Center for Biologics Evaluation and Research, FDA; Anne Eder, MD, PhD, Center for Biologics Evaluation and Research, FDA; Nicole Verdun, MD, Center for Biologics Evaluation and Research, FDA; Tim Uyeki, MD, MPH, MPP, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), USA; Scott Koepsell, MD, PhD, University of Nebraska Medical Center; Annie Winkler, MD, Instrumentation Laboratory; Xuan Qin, PhD, D(ABMM), Seattle Children's Hospital and University of Washington; Toby L. Simon, MD, CSL Behring; Richard J Benjamin, MD, PhD, CERUS Corporation; Jerry A Holmberg, Grifols S A; Vicente Blanquer, Grifols S A; Daniel Fleta, Grifols S A; Amarant Martinez, Grifols S A; and Liu Yang, Grifols S A.
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Nos. 2020‐I2M‐CoV19‐006, 2016‐I2M‐3‐024, and 2017‐I2M‐1‐009).
Contributor Information
Juntao Yang, Email: yangjt@pumc.edu.cn.
Yanyun Wu, Email: yxw1366@med.miami.edu.
Zhong Liu, Email: liuz@ibt.pumc.edu.cn.
REFERENCES
- 1. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med 2020;382:1787‐99. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng YM, Xu XL, He XQ, et al. Comparative effectiveness and safety of ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha and ribavirin plus lopinavir/ritonavir plus interferon‐alphain in patients with mild to moderate novel coronavirus pneumonia. Chin Med J (Engl) 2020;133:1132‐4. 10.1097/CM9.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costanzo M, De Giglio MAR, Roviello GN. SARS‐CoV‐2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem 2020;27:1‐6. [DOI] [PubMed] [Google Scholar]
- 4. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discov Ther 2020;14:58‐60. [DOI] [PubMed] [Google Scholar]
- 5. Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS 2015;10:129‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. John MJ, Maria SC, Kenneth BJ, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis 2015;211:80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garraud O. Use of convalescent plasma in Ebola virus infection. Transfus Apher Sci 2017;56:31‐4. [DOI] [PubMed] [Google Scholar]
- 8. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV Infection, Saudi Arabia. Emerg Infect Dis 2016;22:1554‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hui DS, Lee N, Chan PK, et al. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res 2018;150:202‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA 2020;323:1582. 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . WHO Blood Regulators Network (BRN): Donor selection in case of pandemic situations. 2007 Oct 11 [cited 2020 Apr 5]. Available from: https://www.who.int/bloodproducts/brn/DonorSelectionincaseofPandemicSituations.pdf?ua=1.
- 12. Chen L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis 2020;20:398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roback JD, Guarner J. Convalescent plasma to treat COVID‐19: possibilities and challenges. JAMA 2020;323:1561‐2. [DOI] [PubMed] [Google Scholar]
- 14. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun 2020;11:1620‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science 2020;367:1444‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 2020;367:1260‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du L, Zhao G, He Y, et al. Receptor‐binding domain of SARS‐CoV spike protein induces long‐term protective immunity in an animal model. Vaccine 2007;25:2832‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Hua C, Xia S, et al. Combining a fusion inhibitory peptide targeting the MERS‐CoV S2 protein HR1 domain and a neutralizing antibody specific for the S1 protein receptor‐binding domain (RBD) showed potent synergism against pseudotyped MERS‐CoV with or without mutations in RBD. Viruses 2019;11:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Health Commission of the People's Republic of China . Diagnosis and treatment of new coronavirus pneumonia (trial version 5). [cited 2020 March 13]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf.
- 20. National Health Commission of the People's Republic of China . Technical operation procedures for blood stations (2019 edition). [cited 2020 May 3]. Available from: http://www.nhc.gov.cn/yzygj/s7658/201905/bdd4f4ccd15c4201bfb6d9e7492d7fab/files/9c6c4c3a40a64bf786f5b5d8ee08b220.pdf.
- 21. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg 1938;27:493‐7. [Google Scholar]
- 22. Bin J, Zhang Q, Ge X, et al. Potent human neutralizing antibodies elicited by SARS‐CoV‐2 infection. BioRxiv 2020. (Published article online). 10.1101/2020.03.21.990770. [DOI] [PubMed] [Google Scholar]
- 23. Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016;14:152‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ni L, Ye F, Chen M‐L, et al. Characterization of anti‐viral immunity in recovered individuals infected by SARS‐CoV‐2. medRxiv 2020. (Published article online). 10.1101/2020.03.17.20036640. [DOI] [Google Scholar]
- 25. Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell 2020;181:281‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. New RRC, Moore BD, Butcher W, et al. Antibody‐mediated protection against MERS‐CoV in the murine model. Vaccine 2019;37:4094‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao WC, Liu W, Zhang PH, et al. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med 2007;357:1162‐3. [DOI] [PubMed] [Google Scholar]
- 28. Zhao J, Yang Y, Huang H‐P, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. medRxiv 2020. (Published article online). 10.1101/2020.03.11.20031096. [DOI] [Google Scholar]


