Abstract
Objectives
Many patients with haemoglobinopathies, including thalassaemia and sickle cell disease, are at increased risk of developing severe complications from the coronavirus disease 2019 (COVID‐19). Although epidemiologic evidence concerning the novel coronavirus (SARS‐CoV‐2) infection in these patients is currently lacking, the COVID‐19 pandemic represents a significant challenge for haemoglobinopathy patients, their families and their attending physicians.
Methods
The present statement summarizes the key challenges concerning the management of haemoglobinopathies, with particular focus on patients with either transfusion‐dependent or non‐transfusion‐dependent thalassaemia, identifies the gaps in knowledge and suggests measures and strategies to deal with the pandemic, based on available evidence and expert opinions. Key areas covered include patients’ risk level, adaptation of haemoglobinopathy care, safety of blood transfusions, blood supply challenges, and lifestyle and nutritional considerations.
Conclusions
The proposed measures and strategies may be useful as a blueprint for other disorders which require regular hospital visits, as well as for the timely adaptation of patient care during similar future pandemics.
Keywords: coronavirus, COVID‐19, haemoglobinopathies, pandemic, SARS‐CoV‐2, thalassaemia
Summary Statements.
There is a knowledge gap in the effective management of thalassaemia patients during the COVID‐19 pandemic that this original article addresses. These are mostly regularly transfused patients with frequent hospital visits.
Comprehensive algorithms and patient care pathways in this original article provide a step‐wise methodical depiction of current evidence and expert opinions.
Timely adoption of this original article's suggested considerations could benefit haemoglobinomathy patients, their families and the staff involved in their treatment during this time of need.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has affected millions across the world, having caused hundreds of thousands deaths. 1
Inherited haemoglobin disorders or haemoglobinopathies, including thalassaemias and sickle cell disease (SCD), are the most common monogenic disorders in human, being associated with significant burden, with multisystemic involvement and need for intensive life‐long therapy and follow‐up. 2 , 3 Multiple sequelae often complicate the course of the disease, requiring special care. This is particularly true for patients living in developing or low‐income countries, where disease‐specific management programmes are lacking and access to modern therapy is limited. On the other hand, therapeutic advances of the past decades have resulted in a significant improvement in the once ominous prognosis of thalassaemia patients, and thus, patients with access to modern treatment modalities and well‐organized follow‐up programmes grow old and face a new spectrum of comorbidities related to ageing. 4 As a result, many haemoglobinopathy patients are at increased risk to develop severe complications in the presence of a comorbidity such as a viral infection. At the same time, the significant medical burden of the COVID‐19 pandemic with the unprecedented overload of the healthcare systems across the world and the inevitable diversion of medical resources from other diseases towards COVID‐19 may jeopardize the already demanding and often suboptimal care of patients with haemoglobinopathies.
Despite the fact that epidemiologic evidence concerning SARS‐CoV‐2 infection in patients with thalassaemia and sickle cell disease is currently lacking, 5 the COVID‐19 pandemic represents a significant challenge for haemoglobinopathy patients, their families and their attending physicians. Thalassaemia International Federation (TIF) follows closely the evolution of the COVID‐19 pandemic and compiles continuously the emerging scientific evidence relevant to the care of patients with haemoglobinopathies. The present statement summarizes the key challenges concerning the management of haemoglobinopathies, with particular focus on patients with either transfusion‐dependent or non‐transfusion‐dependent thalassaemia, identifies the gaps in knowledge and suggests measures and strategies to deal with the pandemic based on available evidence and expert opinions provided by members of TIF’s International Scientific Advisory Board. TIF collaborates with international experts in thalassaemia from around the world, many of whom are authors and editors of the clinical management guidelines which are published every four years or so and are the basis of evidence‐based patient care globally. Any document published by TIF is reviewed by at least two such consultants. In addition, other internationally accepted guidelines are consulted; for example the British Society of Haematology guidance on shielding for patients with splenectomy, documents by the UK national haemoglobinopathy panel on COVID‐19, WHO documents and others are regularly followed.
The information provided herein is relevant to patients with (a) homozygous β‐thalassaemia, including thalassaemia major and intermedia; (b) combined forms of thalassaemia, such as β‐thalassaemia/HbE; (c) α‐thalassaemia, particularly HbH, the most clinically significant form. At this stage, sickle cell syndromes will not be further discussed, even though patients affected are expected to be at greater risk of COVID‐19 complications due to the danger of the virus triggering an acute chest syndrome as well as vascular complications. 6 , 7 , 8
Thalassaemia patients stand out in the situation of the pandemic because of their need to frequently visit healthcare facilities for blood transfusion. This makes the need for protection measures imperative as hospital environments may be regarded as “hotspots” for viral transmission. In addition, from TIF contact with patient organization across the world, the pandemic has secondary consequences, such as blood shortages, medication shortages reduced access in some situations to expert care, that may have long‐term effects on the health status.
It should be stressed that as our yet limited knowledge on COVID‐19 progresses, 5 new evidence becomes continuously available that may challenge some of the information and guidance provided herein. The proposed measures and strategies may be useful as a blueprint for other disorders which require regular hospital visits, as well as for the timely adaptation of patient care during similar future pandemics.
2. GENERAL CONSIDERATIONS AND LEVEL OF RISK
The SARS‐CoV‐2 infection presents particular challenges and dangers to patients with haemoglobin disorders. The virus affects primarily the respiratory system, with a disease spectrum ranging from nasopharyngeal symptoms to full blown pneumonia. 9
Generally, these infections can cause more severe symptoms in people with weakened immune systems, older and/or frail people, and those with long‐term conditions like diabetes, cancer and chronic lung disease. 10 Most people (about 80%) who become infected experience mild illness and recover, but it can be more severe for others. 9 Most deaths are related to respiratory complications requiring intensive care and respiratory support, even though an overexuberant inflammatory response with multi‐organ failure may be prevalent in some cases.
So far, very little clinical experience of infected patients with haemoglobin disorders has been recorded. 5 Any statement on these subjects may be regarded as speculations; cautionary thoughts are however necessary, in view of the rapid spread of the virus and the possible factors which may render these patients vulnerable in front of this infection. TIF believes that health services should be alerted to these risks and affected patients warned so that extra precautionary measures can be taken.
Haemoglobin disorders are not directly associated with respiratory conditions. However, disease‐related complications may affect multiple organs including the heart, liver, endocrine glands, lungs and the immune system, thus rendering this patient population at an increased risk to develop serious complications during COVID‐19. 2 , 3 , 11 This is especially so in patients who receive suboptimal management and lack access to modern therapy and multidisciplinary care and/or suboptimal compliance with medical instructions.
Thalassaemia patients do not have the same risk of pulmonary infections with sickle cell disease patients but, they may have multiple organ complications, often due to iron overload, including cardiac and hepatic, diabetes mellitus and endocrine disease. 2 , 3 , 4 One particular endocrine complication, adrenal hypofunction, is often not recognized and may render the patient particularly susceptible to severe infections. This condition requires glucocorticoid supplementation; however, corticosteroids have been shown to slow down the clearance of viral RNA from respiratory tract in patients with SARS‐CoV or MERS‐CoV infections increasing the complications rate. 12
Thalassaemia patients, particularly of the older age groups, have often been splenectomized while SCD patients often suffer from functional hyposplenism or asplenia. This renders these patients vulnerable to bacterial infections that may lead to serious illness or life‐threatening sepsis. When infected by SARS‐CoV‐2, these patients may also develop secondary bacterial infections.
Patients with thalassaemia can be grossly divided into three risk groups: (a) Group A, patients at “moderate risk,” (b) Group B, patients at “high risk” and (c) Group C, patients at “highest risk”; the classification criteria are presented in detail in Table 1. It should be stressed that the proposed risk classification is mainly based on the level of adherence to disease‐specific care, since quality of care is the crucial determinant of morbidity in these patients.
TABLE 1.
Risk levels in patients with haemoglobinopathies and corresponding guidance considerations regarding work, schooling or educational activities
| Risk level | Criteria | Guidance |
|---|---|---|
| Group A: Moderate risk |
Thalassaemia patient with all of the following:
|
Assume work/schooling/education without any additional measures besides national guidelines for general population (distancing, hand washing, wearing mask) |
| Group B: High risk |
Thalassaemia patient with 2 or more of the following:
|
Assume work/schooling/education keeping national guidelines for general population (distancing, hand washing, wearing mask) unless job involves treating/caring patients or other vulnerable groups (eg healthcare professionals) or frequent contact with people (eg receptionists, shop assistants) |
| Group C: Highest risk |
|
Refrain from any type of work/schooling/education activities and remain at home, avoiding any gatherings or contact with potential COVID‐19 (egrelative symptoms) until the pandemic is declared well over at country level. Keep national guidelines of distancing, hand washing and wearing mask at least 6 mo after the pandemic is declared over at country level |
3. ADAPTATION OF HAEMOGLOBINOPATHY CARE
Instructions concerning avoiding SARS‐CoV‐2 infection, reducing spread of the virus, and what to do if infection is suspected are varying from country to country, and therefore, local and national guidance should be followed. 13 , 14 , 15 Adherence to the instructions and recommendations of the national health committees is of pivotal importance and should be incorporated in the care of patients with haemoglobinopathies.
The following are measures that need to be taken in the care of thalassaemia patients.
All patients should comply with the social distancing measures and family practices recommended by local authorities to prevent their exposure to confirmed or possible COVID‐19 cases among their social contacts, including family members or friends.
In patients with symptoms of cough, fever, fatigue or other symptoms suggestive of an acute respiratory illness, it is required to test for COVID‐19 along with other respiratory viral pathogens.
If suspicion for COVID‐19 is high or the test is positive, the treating physician who is fully aware of the individual's care plan should be contacted immediately.
Particularly in those patients with comorbidities, a close collaboration of the attending physician team with the patients’ treating physician is of utmost importance.
Adaptation of thalassaemia care during the present and potential future similar pandemics requires on one hand the strengthening of existing and creation of new communication channels between healthcare professionals and patients and on the other the promotion of a modified patient pathway for access to care including visits to medical facilities. Further modifications are required for the monitoring protocols in order to include additional information and questions related to COVID‐19 pandemic and for the laboratory tests that may need to be rearranged based on prioritization and individual patient needs. All this must be considered but the need for short‐ and long‐term adhesion to patient treatment programmes and protocols to avoid adverse effects must not be neglected.
It is important to stress that all thalassaemia patients should receive proper care regardless of whether they are exposed to or infected by COVID‐19. This can be accomplished by the adoption of appropriate patient care pathways, as described in detail below. In addition, enrolment of thalassaemia patients into COVID‐19 trials should be encouraged as it would generate important evidence for both conditions.
3.1. Communication channels and tools
Sharing of reliable information between healthcare professionals on one hand and patients and their families on the other is crucial during this and other similar pandemics. The strengthening of the existing communication channels and tools and the creation of new ones will facilitate information sharing fulfilling two important aims: (a) to provide information on latest COVID‐19 pandemic updates and advice on necessary distancing or other measures that need to be taken for thalassaemia patients and (b) to ensure appropriate responses to patient queries and timely reporting of symptoms potential related to COVID‐19 (Figure 1).
FIGURE 1.
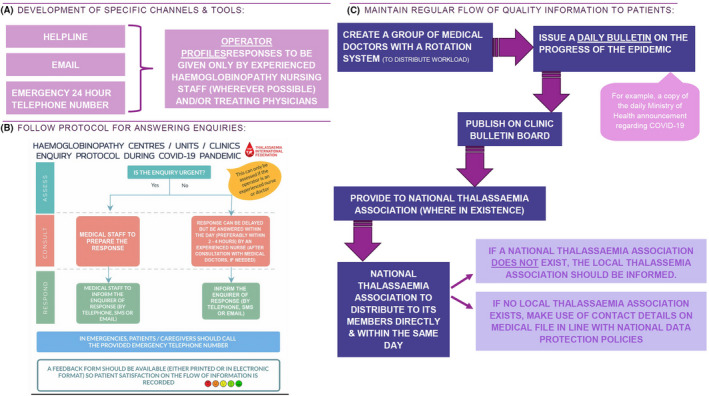
Strategies to enhance information sharing between healthcare professionals and patients and their families by developing communication channel and tools (A), setting up a protocol for answering patient queries (B) and maintaining a regular flow of quality information to patients (C) [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Patient care pathways
To adapt patients’ care to the special conditions and requirements of the COVID‐19 and future similar pandemics, TIF proposes two pathways, one general and one related to transfusions (Figure 2). These pathways have been developed in accordance with the instructions by WHO and other bodies 13 , 14 , 15 and incorporate the necessary changes required in admission of patients to haemoglobinopathy units and clinics, with the aim to protect the individual patient, the medical and other personnel as well as other patients visiting the same medical facilities from possible coronavirus infection. According to these pathways, any visit to the haemoglobinopathy clinic should be appointment‐based only and booked in advance via telephone or online appointment systems. The clinic is advised to carry out a 2‐step triage before any patient enters the facility. A triage nurse, wearing appropriate personal protective equipment 16 will ask the patient a series of questions, as per national guidelines for COVID‐19) and will check the patient's vitals, including temperature, heart rate, respiratory rate, blood pressure and pulse oximetry along with the haemoglobin level. If any COVID‐19‐like symptoms are present such as high temperature or dry cough, the triage nurse will consult with the treating physicians to decide upon granting admission to the clinic or not. A proposed algorithm for the screening for COVID‐19 in patients with haemoglobinopathies is presented in Figure 3. In the case that admission is permitted to the patient with COVID‐19‐like symptoms, he/she should receive the required medical care in a designated “isolation room” by doctors and nurses wearing appropriate personal protective equipment. 16 The haemoglobinopathy units and clinics are thus advised to designate a dedicated room within the medical facility that will be used to provide medical care to thalassaemia patients who are suspected or confirmed COVID‐19 patients. This room should ideally have a separate entrance and exit to the rest of the clinic so as to limit contact with other patients, thus preventing transmission of COVID‐19. The room should be disinfected regularly.
FIGURE 2.
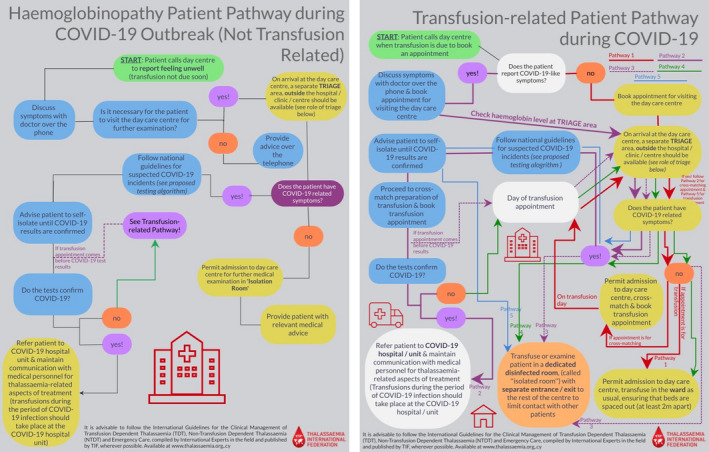
Patient pathways during the pandemic: general pathway (left panel) and transfusion‐dependent patient pathway (right panel) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
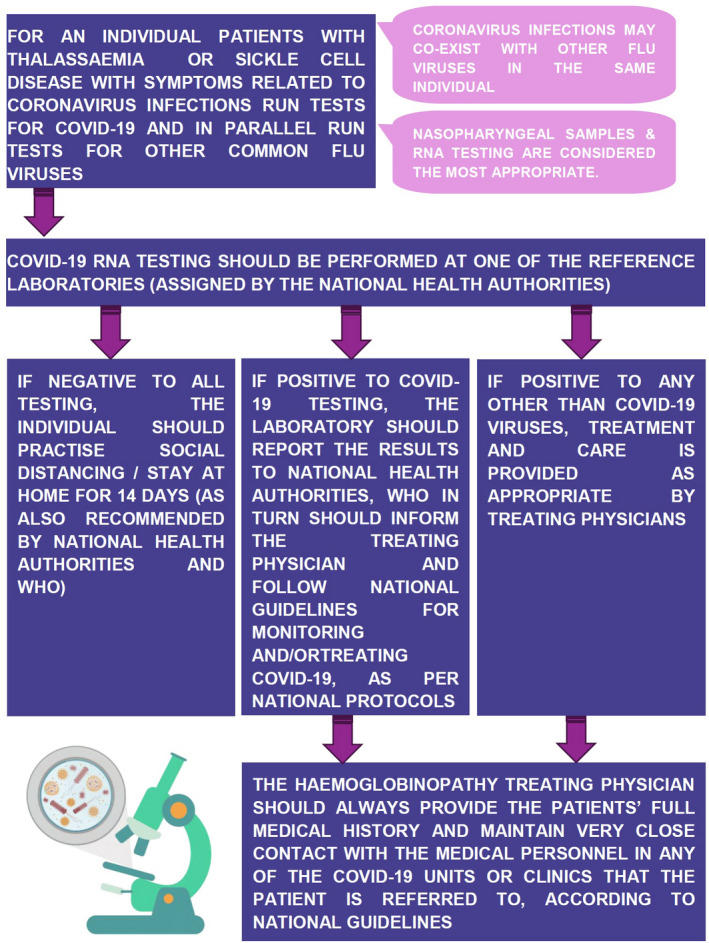
A proposed algorithm for the diagnosis of COVID‐19 in patients with haemoglobinopathies [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Monitoring tests and medications
The routine tests that are crucial for the monitoring of thalassaemia patients including full blood count, pretransfusion haemoglobin levels and serum ferritin should continue as usual for the duration of the COVID‐19 epidemic, in accordance with the precautions outlined in the patient care pathways, describe (Figure 2). Annual routine monitoring tests including magnetic resonance imaging (MRI), Ferriscan, dual‐energy X‐ray absorptiometry (DEXA), ophthalmology & audiology tests should better be postponed until after the end of the pandemic in clinically stable patients with low iron load and no new indications for these tests, unless the treating physician advises otherwise.
As previously stressed, the patients should generally be advised to follow the self‐protection and social distancing measures issued by the local health authorities. The patients should also be instructed not to discontinue any medication before consulting with their treating physician, including the renin‐angiotensin‐aldosterone inhibitors, in accordance with the recommendations of scientific associations and bodies. 17 , 18
4. SAFETY OF BLOOD TRANSFUSIONS
Despite the fact that coronavirus RNA can be amplified from patients’ blood, there is no hitherto evidence of coronavirus transmission through transfusion of blood and blood products. 19 All respiratory viruses (except for adenoviruses), including coronavirus, normally attach to receptors in the airways and their blood‐borne transmission is unknown or at least very confined. Low to very low viraemia has been detected in some symptomatic patients, although viraemia in the incubation period, asymptomatic course of infection and after symptoms’ resolution has not been documented. Transmission of other ‘similar’ viruses, SARS‐CoV, MERS‐CoV and other coronaviruses through transfusion or transplantation has not been reported to date. 20 In this regard, the WHO, the AABB, the FDA and the US CDC mention that competent authorities and blood establishments do not currently require to take any actions as there is no data suggesting a risk of transmission of SARS‐CoV2 through transfusion. 21 , 22 , 23 , 24 SARS‐CoV‐2 may be infectious during an asymptomatic incubation period and precautions are relevant in this regard.
The strengthening of donor selection criteria is of utmost importance. The careful screening of donors, with particularly focus on COVID‐19‐related symptoms and signs, is expected to prevent symptomatic cases from blood donation. In the case of confirmed COVID‐19 patients, donation should be deferred for at least 28 days after symptoms resolution and completion of therapy. Addition of stricter questions in the donor questionnaire can enhance deferral of asymptomatic SARS‐CoV‐2 carriers. A precautionary deferral of blood donation for 21 days is recommended following exposure to a confirmed patient. 25
A donor follow‐up system is necessary during the pandemic. 21 This could be accomplished through a well‐structured postdonation questionnaire that would report donors’ health status consistent with COVID‐19 for at least 14 days and until 28 days after donation. Blood and components collected within 14 to 28 days of disease onset in the donor may be recalled as a precautionary measure. 21 , 26
The routine practices for donor management and infectious disease testing should not be changed. However, in extreme blood shortage, reduction of whole blood donation intervals may be considered for donors with robust haemoglobin level who are able to tolerate more frequent donations. 21
Screening of the blood and blood product supplies for SARS‐CoV‐2 is not currently recommended in the absence of documented transfusion transmission, demonstrated infectivity of SARS‐CoV‐2 in blood collected from asymptomatic persons or availability of a nucleic acid testing (NAT) for blood screening specific for this virus. 21
Pathogen Reduction technologies (PRT) are demonstrated to be effective against SARS‐CoV and MERS‐CoV in plasma and platelets and are likely to be effective for SARS‐CoV‐2. Such methods, however, require significant logistical and financial investment they are not yet available for red blood cells.
Robust haemovigilance programmes should be in place to capture any possible cases of viral transmission while facilitating the understanding the behaviour of the virus. 21 Such programmes are however in place only in a small number of countries and mainly in the Western World.
The optimization of blood use will help safeguard blood supplies during the pandemic. 21 In this context, adoption of a patient blood management (PBM) system, based on three pillars, optimization of patient's own blood volume, minimization of blood loss and harnessing the physiological anaemia reserve, reduces the need for transfusions, improves patient outcomes and reduces length of hospital stay and costs. 27 One particular aspect of optimizing blood usage could be to the use of erythropoiesis stimulants, with a view to reducing transfusion requirements. Currently, this is possible through various approaches with varying levels of success, including the use of hydroxyurea, 28 enhancers of HbF production, 29 and the newer approaches using red cell maturation modifiers such as the recently approved luspatercept. 30 These interventions are likely to benefit few patients, however, since responses are indeed unpredictable or the medications inaccessible.
Arrangements to facilitate donors are necessary through the use of mobile units, and above all the creation of a truly safe environment for the donors and the staff involved are crucial. In addition, the provision of updated information on the daily progress of the pandemic may further help empowering people to donate. 31
For plasma derivatives on the other hand, regular screening of donors showing clinical symptoms and the established processes of virus inactivation and removal during manufacturing, as has been demonstrated with other large size lipid‐enveloped RNA viruses, should be sufficient to mitigate COVID‐19 transmission through plasma derivatives. Therefore, the COVID‐19 outbreak is not considered a threat to the safety of plasma therapies when the above methods are in place.
COVID‐19 has not been described in organs or haematopoietic stem cell transplant (HSCT) recipients to date. An additional challenge in transplanted patients is that due to their immunosuppression status, they may have prolonged shedding of the virus which increases the risk of transmission to their contacts including health care professionals. Conversely, immunosuppressed transplant recipients who regularly visit hospital settings may be exposed to the SARS‐CoV‐2 in hospital settings with a higher possibility to develop severe illnesses.
5. BLOOD SUPPLY CHALLENGES
The influenza A virus subtype H1N1 (H1N1) pandemic had a significant impact on the blood supply due to donors’ fear of exposure to the virus at a hospital or a free‐standing donor facility. 32 Similarly, the COVID‐19 pandemic has already led to reduced blood supplies due to the cancellation of numerous community‐based and mobile blood donation drives, as well as a marked reduction in donors arriving for scheduled appointments. 31 In the US, nearly 4000 American Red Cross blood drives, that account for more than 80% of its blood collects, have been cancelled across the country, 33 while cancellation of hospital‐based collections resulted in 130 000 fewer donations in only a few weeks. 27
Besides the cancellation of organized blood donation drives and campaigns and the significant reduction in the number of donors arriving at blood donation facilities due to self‐quarantine, social isolation measures and the fear of exposure to COVID‐19, there is further the inevitable deferral of donors due to the more meticulous donor selection process with additional criteria discussed above. The situation further worsens by the fact that an increasing number of staff members in the blood services become ill or are required to self‐isolate, while part of the blood donation and processing procedures may potentially be diverted to the preparation of convalescent plasma from patients who have recovered from COVID‐19. Finally, the blood supply chain can be affected by travel restrictions as well as factory closing that may affect the blood product testing and production. At a later stage of the pandemic, a considerable percentage of the population is expected to be unknowingly infected by SARS‐CoV‐2, including the young blood donor population in which asymptomatic cases are more common; this will render the deferral of potential asymptomatic affected donors much more challenging.
On the other hand, the demand for blood and components may decrease during the pandemic as elective and non‐urgent surgical interventions are deferred and road and other society‐related accidents may decrease due to isolation measures. This may compensate to a certain extend the anticipated reduction of blood supplies for chronically transfusion‐dependent patients.
6. LIFESTYLE AND NUTRITIONAL CONSIDERATIONS
In addition to the well‐known personal hygiene and preventive measures against the new coronavirus, thalassaemia patients can also follow some simple recommendations regarding their lifestyle and nutrition that strengthen their immune system and could better prepare them for the ongoing pandemic. 34 , 35
These recommendations are based on the implementation of common sense and moderation, and consist of:
Sufficient hydration with 2‐3 litres of water consumed throughout the day, if health conditions allow it.
Adequate rest with 7‐8 hours of sleep daily.
Regular but not exhaustive exercise with a half‐hour walk, 3 days a week.
Normal body weight maintenance through a well‐balanced diet. Especially during the pandemic patients should consume frequent small and light meals, mainly consisting of fruits and vegetables.
Effective stress management through calm mindfulness.
Continuous management of chronic comorbidities, especially cardiac and pulmonary conditions, and diabetes mellitus.
Self‐disciplined smoking cessation as even otherwise healthy smokers are considered of high risk for suffering severe complications from COVID‐19.
Controlled brief exposures to direct sunlight for mood boosting, sleep regulation, as well as for essential vitamin D production.
Regarding nutrition in particular, thalassaemia patients are encouraged to consume natural sources containing vitamin C (citrus fruits), vitamin D (sardine, mackerel, dairy products), zinc (legumes, seeds and nuts) and omega‐3 fatty acids (sardine, mackerel) as these foods have been found in applied basic research to provide necessary vitamins, minerals and anti‐oxidants that boost the immune response. Caution is advised if patients turn to dietary supplements as the danger of over‐supplementation (especially for vitamin C) is quite considerable. While there are no clinical data available there is a belief that probiotics (especially preparations with lactobacilli and bifidobacteria species) could help against most viral infections, COVID‐19 included, and their use towards this direction should be further investigated given their rather safe profile.
Other potentially beneficial phytochemicals against the coronavirus infection, with strong preclinical evidence but not actually tested on human subjects in randomized clinical trials, include procyanidins, 36 lectins, 36 , 37 luteolin, 36 hesperetin, 37 catechins and sulforaphanes. They are all found within a flavonoid‐rich plant‐based diet. Caution is again advised regarding possible food‐drug interactions.
7. CONCLUSIONS
The clinical impact of COVID‐19 in haemoglobinopathy patients is not yet defined and thus infected cases should be under meticulous observation with comprehensive reporting of their clinical outcomes. This will contribute to the prompt management of the potential complications that may arise in infected patients, but also to the collection and sharing with other treating physicians of important information and data that will support the better understanding of the infection in this special population. In this context, TIF is collaborating with international medical experts for developing a survey on the clinical outcomes of haemoglobinopathy patients with confined COVID‐19. In addition, the current pandemic stresses the importance of patient databases and registries in obtaining reliable disease data and inform healthcare policies. Unfortunately, the majority of thalassaemia patients live and receive care in developing or low‐income countries, where there is a general lack of patient databases. To address this unmet need, TIF has undertaken a series of relevant initiatives, including the recent development of an international web‐based registry platform.
Farmakis D, Giakoumis A, Cannon L, Angastiniotis M, Eleftheriou A. COVID‐19 and thalassaemia: A position statement of the Thalassaemia International Federation. Eur J Haematol. 2020;105:378–386. 10.1111/ejh.13476
REFERENCES
- 1. World Health Organization (WHO) . WHO COVID‐19 Dashboard. https://who.sprinklr.com. Accessed May 12, 2020
- 2. Thalassaemia International Federation . Guidelines for the Management of Transfusion Dependent Thalassaemia, 3rd ed. Nicosia, Cyprus: Thalassaemia International Federation; 2014. [Google Scholar]
- 3. Thalassaemia International Federation . Guidelines for the Management of Non‐Transfusion Dependent Thalassaemias, 2nd ed. Nicosia, Cyprus: Thalassaemia International Federation; 2017. [Google Scholar]
- 4. Farmakis D, Giakoumis A, Angastiniotis M, Eleftheriou A. The changing epidemiology of the ageing thalassaemia populations: a position statement of the Thalassaemia International Federation. Eur J Haematol. 2020;105(1):16‐23. 10.1111/ejh.13410 [DOI] [PubMed] [Google Scholar]
- 5. Motta I, Migone De Amicis M, Pinto VM, et al. SARS‐CoV‐2 infection in beta thalassemia: preliminary data from the Italian experience. Am J Hematol. 2020; 10.1002/ajh.25840 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza‐related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125(2):234‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beerkens F, John M, Puliafito B, Corbett V, Edwards C, Tremblay D. COVID‐19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hematol. 2020;95(7):E154‐E156. 10.1002/ajh.25809 [DOI] [PubMed] [Google Scholar]
- 8. Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol. 2020;95(6):725‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farmakis D, Giakoumis A, Polymeropoulos E, Aessopos A. Pathogenetic aspects of immune deficiency associated with beta‐thalassemia. Med Sci Monit. 2003;9(1):RA19‐22. [PubMed] [Google Scholar]
- 12. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Coronavirus disease (COVID‐19) Pandemic. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed April 14, 2020
- 14. Centers for Disease Control and Prevention . Coronavirus (COVID‐19). https://www.cdc.gov/coronavirus/2019‐ncov/index.html. Accessed April 14, 2020
- 15. European Centre for Disease Prevention and Control . Coronavirus COVID‐19. https://www.ecdc.europa.eu/en/coronavirus. Accessed April 14, 2020
- 16. World Health Organization . Rational use of personal protective equipment (PPE) for coronavirus disease (COVID‐19). https://apps.who.int/iris/handle/10665/331498. Accessed April 14, 2020
- 17. Heart Failure Society of America / American College of Cardiology / American Heart Association . Statement Addresses Concerns Re: Using RAAS Antagonists in COVID‐19. https://www.acc.org/latest‐in‐cardiology/articles/2020/03/17/08/59/hfsa‐acc‐aha‐statement‐addresses‐concerns‐re‐using‐raas‐antagonists‐in‐covid‐19. Accessed April 14, 2020
- 18. Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020; 10.1093/cvr/cvaa097 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang L, Zhao L, Gong H, Wang L, Severe WL. Acute respiratory syndrome Coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26(7):1631‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Society of Blood Transfusion . Webinar: Update on the COVID‐19 Coronavirus Outbreak: Blood Collection and Safety Implications. https://education.isbtweb.org/isbt/2020/covid‐19/289245/michael.busch.louis.m.katz.26.hua.shan.webinar.update.on.the.covid‐19.html . Accessed April 14, 2020.
- 21. World Health Organization . Maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID‐19). https://www.who.int/publications‐detail/maintaining‐a‐safe‐and‐adequate‐blood‐supply‐during‐the‐pandemic‐outbreak‐of‐coronavirus‐disease‐(covid‐19). Accessed April 14, 2020
- 22. World Health Organization . Protecting the Blood Supply During Infectious Disease Outbreaks. https://www.who.int/bloodsafety/publications/protecting‐blood‐supply/en/. Accessed April 14, 2020
- 23. American Association of Blood Banks . AABB’s Resources for: FDA’s Updated Information for Blood Establishments Regarding the Novel Coronavirus (COVID‐19) Outbreak. http://www.aabb.org/advocacy/regulatorygovernment/Documents/COVID‐19‐Toolkit.pdf. Accessed April 14, 2020
- 24. U.S. Food & Drug Administration . Updated Information for Blood Establishments Regarding the Novel Coronavirus Outbreak. https://www.fda.gov/vaccines‐blood‐biologics/safety‐availability‐biologics/updated‐information‐blood‐establishments‐regarding‐novel‐coronavirus‐outbreak. Accessed April 14, 2020
- 25. European Centre for Disease Prevention and Control . Coronavirus disease 2019 (COVID‐19) and supply of substances of human origin in the EU/EEA. https://www.ecdc.europa.eu/en/publications‐data/coronavirus‐disease‐2019‐covid‐19‐and‐supply‐substances‐human‐origin‐eueea. Accessed April 14, 2020
- 26. Kwon SY, Kim EJ, Jung YS, Jang JS, Cho NS. Post‐donation COVID‐19 identification in blood donors. Vox Sang. 2020; 10.1111/vox.12925 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Shander A, Goobie SM, Warner MA, et al. The essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020;131(1):74‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Algiraigri AH, Wright NAM, Paolucci EO, Kassam A. Hydroxyurea for nontransfusion‐dependent β‐thalassemia: a systematic review and meta‐analysis. Hematol Oncol Stem Cell Ther. 2017;10(3):116‐125. [DOI] [PubMed] [Google Scholar]
- 29. Musallam KM, Taher AT, Cappellini MD, Sankaran VG. Clinical experience with fetal hemoglobin induction therapy in patients with β‐thalassemia. Blood. 2013;121(12):2199‐2372. [DOI] [PubMed] [Google Scholar]
- 30. Cappellini MD, Viprakasit V, Taher AT, et al. A phase 3 trial of luspatercept in patients with transfusion‐dependent β‐thalassemia. N Engl J Med. 2020;382(13):1219‐1231. [DOI] [PubMed] [Google Scholar]
- 31. U.S. Food & Drug Administration . Alternative procedures for blood and blood components during the COVID‐19 public health emergency: guidance for industry. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/alternative‐procedures‐blood‐and‐blood‐components‐during‐covid‐19‐public‐health‐emergency. Accessed April 14, 2020
- 32. Institute of Medicine (US) Forum on Microbial Threats . The Domestic and International Impacts of the 2009–H1N1 Influenza A Pandemic: Global Challenges, Global Solutions: Workshop Summary. https://www.ncbi.nlm.nih.gov/books/NBK52789/. Accessed April 14, 2020 [PubMed]
- 33. American Red Cross . Coronavirus Outbreak: Help Us Continue to Deliver Our Lifesaving Mission Nationwide Due to This Public Health Emergency. https://www.redcross.org/content/dam/redcross/about‐us/disaster‐relief/coronavirus‐outbreak‐donor‐info‐sheet‐3‐18‐20.pdf. Accessed April 14, 2020
- 34. Thalassaemia International Federation . A useful health & nutrition short guide for the COVID‐19 pandemic by TIF. https://thalassaemia.org.cy/news/a‐useful‐health‐nutrition‐short‐guide‐for‐the‐covid‐19‐pandemic‐by‐tif. Accessed April 14, 2020
- 35. Alschuler L, Weil A, Horwitz R, et al. Integrative considerations during the COVID‐19 pandemic. Explore (NY). 2020;S1550‐8307(20)30113‐0. 10.1016/j.explore.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stephen Harrod Buhner . Herbal Antivirals: Natural Remedies for Emerging & Resistant Viral Infections. Viral Respiratory Infections and Their Treatment. SARS and Coronaviruses. North Adams, MA: Storey Publishing; 2013:54‐57. [Google Scholar]
- 37. De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti Infect Ther. 2006;4(2):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]


