Abstract
Prone positioning is used for surgical access and recently in exponentially growing numbers of coronavirus disease 2019 patients who are ventilated prone. To reduce their facial pressure ulcer risk, prophylactic dressings can be used; however, the biomechanical efficacy of this intervention has not been studied yet. We, therefore, evaluated facial soft tissue exposures to sustained mechanical loads in a prone position, with versus without multi‐layered silicone foam dressings applied as tissue protectors at the forehead and chin. We used an anatomically realistic validated finite element model of an adult male head to determine the contribution of the dressings to the alleviation of the sustained tissue loads. The application of the dressings considerably relieved the tissue exposures to loading. Specifically, with respect to the forehead, the application of a dressing resulted in 52% and 71% reductions in soft tissue exposures to effective stresses and strain energy densities, respectively. Likewise, a chin dressing lowered the soft tissue exposures to stresses and strain energy densities by 78% and 92%, respectively. While the surgical context is clear and there is a solid, relevant need for biomechanical information regarding prophylaxis for the prone positions, the projected consequences of the coronavirus pandemic make the present work more relevant than ever before.
Keywords: COVID‐19, finite element modelling, pressure injury, prone position, silicone foam multi‐layered prophylactic dressings
1. INTRODUCTION
A pressure ulcer (PU), also called a pressure injury, is localised damage in soft tissues that are subjected to sustained mechanical loading, often by bodyweight forces. 1 Patients who are stationary, paralysed, or under anaesthesia endure prolonged pressures and shear loads at contact sites between their body and support surfaces, which over time, may cause PUs. 2 , 3 , 4 The PU risk increases 3.3‐fold when insensate patients are placed prone, with respect to a supine position. 5 Facial PUs are among the most common anatomical sites for PUs associated with prone positioning, which is typically used in all spinal surgeries, in large‐volume liposuctions, in nephrolithotomy (for treating large or complex renal stones) and where surgical access to the posterior head and neck is required. 6 Intraoperatively‐acquired PUs are reported to occur 5% to 66% of the times, correlating to an increase in the length of hospital stays by 6.7 days for those patients affected by PUs, therefore, adding approximately $1.3‐billion in annual healthcare costs in the United States alone. 6 , 7 , 8 , 9 , 10 The longer the surgery time is, the greater the risk of an intraoperatively acquired PU. 11 In addition to surgery, the use of prone positioning is also rising sharply in intensive care units (ICUs) worldwide, where mechanically ventilated patients with acute respiratory distress syndrome (ARDS) resulting from the coronavirus 2019 disease (COVID‐19) are placed prone. Prone ventilation sessions of approximately 9 to 24 hours (depending on the practice across different facilities and patient conditions) appear to improve the chest wall mechanics, lung recruitability, and tissue oxygenation in ARDS COVID‐19 patients and to lower their risk of ventilator‐induced lung injuries. 12 , 13 , 14 Clearly, however, these COVID‐19 patients are also susceptible to facial PUs during the prone ventilation sessions, which is highlighted in new international clinical guidelines published since the outbreak of the pandemic. 15 , 16
Prone positioning is associated with several important and potentially catastrophic complications, such as haemodynamic changes, 17 , 18 , 19 , 20 ophthalmologic and neurological complications, 21 , 22 , 23 , 24 , 25 , 26 , 27 visual loss, 6 , 8 , 17 , 28 , 29 and of course, PUs. 6 , 8 , 9 , 17 , 30 , 31 , 32 , 33 , 34 During prone surgical procedures, the primary support areas of the head are the forehead and chin, especially for patients positioned on an Andrews frame for prolonged spine procedures. 31 , 33 In addition to the muscle flaccidity and hypotensive effects associated with anaesthesia and the blood loss during surgery, the face has little muscle mass to provide blood supply to the skin and subcutaneous facial tissues under the sustained deformations caused by the weight of the prone head. This promotes the development of facial PUs, particularly at the primary support regions of the prone head, that is, the forehead and chin. 8 , 28 The overall thin facial tissue structures imply that the sustained deformations would have a damaging effect that extends beyond the skin, and indeed, there is anecdotal evidence that some of the facial PUs associated with a prone position is deep tissue injuries (DTIs). 7 , 35 , 36 , 37 , 38 , 39 The DTI nature of these facial injuries (which are difficult to heal without significant scarring compared with category 1/2 PUs 35 ) requires a more comprehensive bioengineering approach to protect the facial soft tissues in prone positioning. Specifically, an adequate prophylactic intervention targeting the forehead and chin should have good biomechanical efficacy in alleviating both the superficial and deep facial soft tissue loads.
Typically, pressure ulcer prevention (PUP) involves routine skin assessments, frequent off‐loading of pressure points, and repositioning. Frequent off‐loading and repositioning reduce pressures, frictional forces, and shear in soft tissues, however, these interventions can pose a challenge in patients who are critically ill or treated prone. 40 The anaesthesia delivered to surgical and to ventilated ICU patients neutralises their ability to move spontaneously or otherwise respond to or report the discomfort and pain that precede tissue damage in PU formation. From a PUP perspective, the option of repositioning prone patients is either non‐existent – that is, during surgery ‐ or very limited, for the ARDS COVID‐19 patients who are mechanically ventilated and connected to a variety of monitors, probes, and tubing. Only small micro‐shifts to offload pressure are recommended during the prone period due to the risk of dislodgement of tubes and devices and concerns over the loss of the physiological benefit of proning. 41 , 42 In addition, moving ARDS COVID‐19 patients into and out of the prone position is labour‐intensive, and requires multiple highly trained nursing staff to coordinate the positioning safely. For patients in the prone position, alternative approaches to reducing facial tissue loads should, therefore, be considered. Prophylactic intervention alternatives for lowering the risk of facials PUs in such scenarios, therefore, reduce to conducting regular skin assessments, skin hygiene regimens, and redistributing the contact loads by means of head positioners and prophylactic dressings. 1 , 7 , 15 , 17 , 30 , 31 , 43 , 44 With reference to the latter, perioperative nursing guidelines highlight the protective benefits in using dressings prophylactically to pad the eyes and facial tissues during prone surgeries, 43 similarly to the practice of protecting other at‐risk areas using dressings in supine procedures (ie, the sacrum and heels). 4 , 7 , 15 , 17 , 43 , 45 , 46 Also relevant is the common nursing practice of protecting the bridge of the nose using dressings cut to shape, prior to continuous positive airway pressure treatments in order to cushion the mask‐face interface. 47 Nevertheless, the biomechanical efficacy of prophylactic dressings in protecting facial weight‐bearing sites during prone positioning has not been studied so far.
Here we addressed the above problem for the first time by investigating the biomechanical performances of the Mepilex Border Flex dressing design (Mölnlycke Health Care, Gothenburg, Sweden) in protecting facial tissues while the head is prone. The aforementioned dressing belongs to the multi‐layered silicone‐foam dressing family; however, it is unique in offering considerable flexibility and extensibility, which are achieved through repeated patterns of Y‐shaped cuts in its retention and spreading layers. The flexibility of the dressing structure facilitates the management of multi‐directional bodyweight‐related forces. In theory, a substantial portion of the mechanical energy associated with such forces, particularly shearing forces acting at the plane of the Y‐cuts, should already be absorbed in the dressing structure through spreads of the cuts and, therefore, that energy does not reach the tissues.
To test this theory, and evaluate how the above dressing interacts with the facial tissues of a prone head, we used a computational anatomically realistic three‐dimensional (3D) head model, which considers the shape, composition, and mechanical behaviour of both the tissues and the applied dressings. The results of the present modelling work allowed to isolate the specific contributions of the studied dressing to the alleviation of forehead and chin tissue loads in prophylactic use. This information is critically needed for protecting surgical and ICU prone patients, including the ARDS COVID‐19 population, from devastating facial PUs.
2. METHODS
In this work, two comparable 3D, anatomically accurate computational finite element (FE) model configurations of the adult human head were developed (Figure 1). These two head model configurations were employed for providing quantitative information regarding potential differences in facial skin and subcutaneous fat loading during a prone surgical or intensive care position, with or without prophylactic dressing protection. The dressings, of the Mepilex Border Flex type, were applied in one model configuration as tissue protectors at the primary face‐support contact areas, the forehead, and chin. The head model without the dressings was used as a reference case, for comparison.
FIGURE 1.
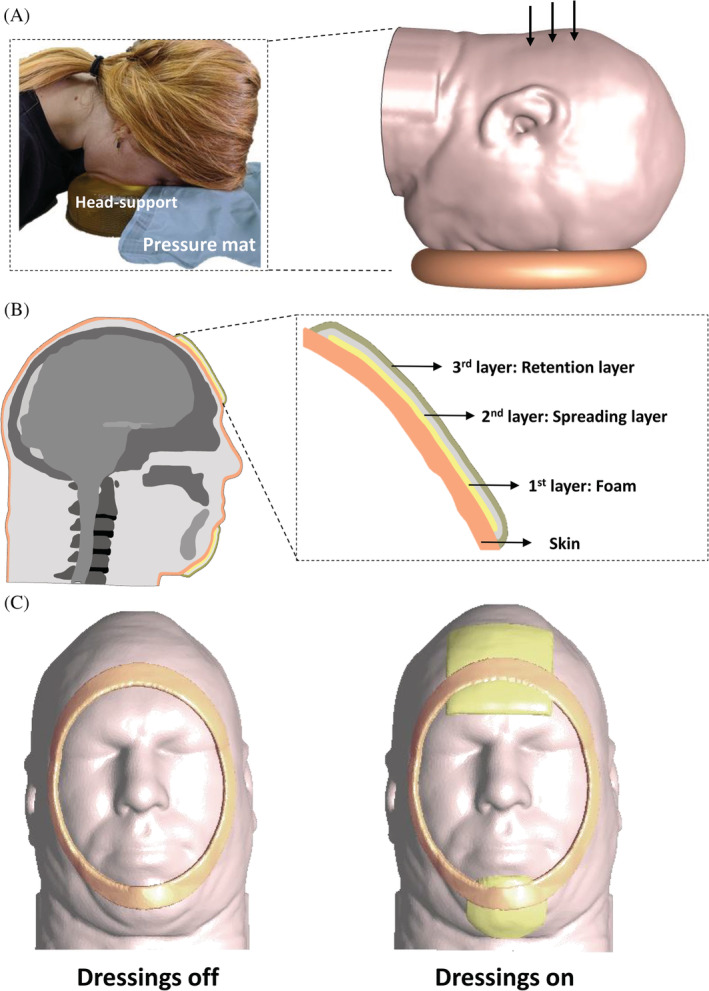
The model geometry and determination of boundary conditions: A, The three‐dimensional (3D) anatomically‐accurate computational finite element (FE) model of an adult head in a prone surgical or intensive care position. The frame on the left‐hand side documents measurements of facial interface pressures when the head of a prone subject is positioned on a donut‐shaped headrest. B, Mid‐sagittal cross‐section through the 3D FE head model with magnification to visualise the structure of the multi‐layered Mepilex Border Flex dressing (Mölnlycke Health Care, Gothenburg, Sweden) which is simulated to be applied prophylactically here, to protect the forehead (in the magnified cross‐section) and chin. C, Inferior views of the 3D FE head model when positioned on the donut‐shaped headrest with the applied forehead and chin dressings (right frame) and without dressings (left frame). The contours of the head support are also shown in both cases, for clarity
2.1. Geometry
Both FE model configurations employed the same male adult head geometry that was built using the visible human (male) project image database (Figure 1) 48 and, which has been investigated in multiple studies published by our group, for example, concerning the risk of a device‐related PU while wearing an oxygen mask. 44 , 47 , 49 , 50 Tissues in each transverse slice of the head model were segmented and then unified to create a 3D geometrical reconstruction using the Scan‐IP module of the Simpleware segmentation software package. 51 The dimensions of the head were 16.5 cm ear‐to‐ear and 21.5 cm occiput‐to‐forehead. 52 In order to simulate a prone surgical or intensive care position, the geometry of a generic donut‐shaped headrest, of the type often used for prone positioning in the above clinical settings, was also created using the Scan‐IP module of Simpleware. In addition, in one model configuration, we generated the aforementioned multi‐layered dressings at the forehead and chin with their detailed layered structure as shown in Figure 1B.
2.2. Mechanical properties of the model components
In our general head modelling framework reported in, 50 the anatomical details of the brain, sinuses, optic nerves, eyeballs, and other soft tissue structures have been included. 50 However, in the present study, these neural and ophthalmic tissues do not influence the facial soft tissue loads. Therefore, we provide here only the mechanical properties of the relevant tissues of the head model for simulating prone positioning, that is, skin, subcutaneous fat, and skull tissues, 47 , 50 which were adopted from experimental work reported in the literature (Table 1). Specifically, the bone tissue of the skull was modelled as a homogeneous, linear‐elastic, and isotropic material with an elastic modulus of 6484 MPa and a Poisson's ratio of 0.2. Facial skin and fat tissues were also assumed to be homogeneous and isotropic but were considered as non‐linear‐elastic materials, which undergo large deformations during head weight‐bearing. Accordingly, these soft tissues were represented using a Mooney‐Rivlin material model with a strain energy density (SED) function (W) as follows:
where I 1 and I 2 are the first and second invariants of the right Cauchy‐Green deformation tensor, respectively, and J is the determinant of the deformation gradient tensor.
TABLE 1.
Mechanical properties and element data for all the finite element model components
| Model component | Shear modulus [MPa] | Bulk modulus [MPa] | Elastic modulus [MPa] (E) | Poisson's ratio (v) | Number of mesh elements | |
|---|---|---|---|---|---|---|
| Skin a | 0.031900 | 3.1794 | — | — | 129 413 | |
| Fat b , c | 0.000286 | 0.0285 | — | — | 341 956 | |
| Skull d | — | — | 6483.6 | 0.495 | 159 263 | |
| Donut‐shaped headrest | — | — | 0.08 | 0.495 | 21 363 |
| E x | E y | E z | v xy | v yz | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dressing (by layer) | Border | — | — | 0.18 | 0.09 | 0.006 | 0.43 | 0.43 | 24 048 |
| Non‐woven | 0.88 | 0.44 | 0.029 | 0.05 | 0.13 | 19 296 | |||
| Polyurethane foam | 0.14 | 0.07 | 0.005 | 0.3 | 0.3 | 13 830 |
Following the same computational methodology reported in our previous published work concerning FE simulations of the use of sacrum and heel prophylactic dressings, 45 , 46 , 53 , 54 , 55 , 56 , 57 , 58 , 59 the layers of the dressings applied here (Figure 1B) were all considered elastic materials. The tensile elastic moduli associated with stretching of the dressing materials during weight‐bearing of the head in the prone position (E x, E y), the compressive elastic moduli of the dressing layers (E z) and the Poisson's ratios were calculated. The pattern of the cuts embedded within the dressing structure was represented in the modelling through its effective contribution to the flexibility and low stiffness of the dressing (ie, the shape details of the individual Y‐cuts were not incorporated directly). These material property data are summarised in Table 1. Lastly, the donut‐shaped headrest was considered as a homogeneous linear‐elastic isotropic material with an elastic modulus of 80 kPa and a Poisson's ratio of 0.495, based on the literature 60 (Table 1).
2.3. Boundary and material interface conditions
We simulated weight‐bearing of the head on the donut‐shaped headrest in a prone position, which represents surgical and intensive care prone positioning. For this purpose, we applied a perpendicular force of 40 N (~4 kg, which represents the weight of an adult male head) at the back of the modelled head, to push the head against the headrest, thereby simulating weight‐bearing. Contacts between the facial skin and headrest, between the skin‐facing side of the dressings and the skin, and between the outer dressing surfaces and the headrest were all set as “tie.” Likewise, the skin‐fat and fat‐skull material interfaces were defined as “tie.”
2.4. Numerical method
Both head model configurations (with and without the dressings) were meshed using the Scan‐IP module of Simpleware. All elements were of the tetrahedral type; the numbers of elements in each model component are specified in Table 1. The simulations were solved using the Pardiso FE solver (version 2.5) and post‐processed using PostView (version 1.10.2), which are both FEBio modules (University of Utah, Salt Lake City, Utah). 61 , 62 The runtime of each model configuration was approximately 2 hours, using a 64‐bit Windows 7‐based workstation with a central processing unit (CPU) comprising 2 Intel Xeon E5645 processors at a clock speed of 2.40 GHz and 64 GB RAM.
2.5. Validation with respect to facial interface pressure measurements
For validation purposes, we measured the interface pressures between the foreheads of prone subjects (two females, one male, at ages of 26, 34, and 26 years, respectively, and bodyweights of 43, 52, and 81 kg, respectively) and a donut‐shaped headrest, using a pressure mat system (M‐flex model, Vista Medical Europe B.V., Maasbree, the Netherlands) (Figure 1A). Dressings were not applied. Pressure measurements were acquired 1 minute after subjects were placed in the prone position. Peak and average forehead pressures were 6.84 ± 3.64 kPa and 2.4 ± 1.18 kPa, respectively (mean ± SD), in good agreement with the corresponding forehead pressure data calculated from the FE simulation of the weight‐bearing head model with no dressings. We attempted to use the same method for the chin, but it appeared that the anatomical contours were too curved for adequate interface pressure acquisition at that location.
2.6. Data analysis and outcome measures
The method of analysis of the data provided by the present FE simulations follows our previous published work where it is described in detail. 44 , 47 , 49 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 63 A brief explanation is provided here for completeness. First, we calculated the distributions of effective stresses and SEDs in the skin and subcutaneous fat tissues. Next, we plotted the volumetric exposures of skin and fat tissues to effective stresses and SEDs in the two relevant anatomical volumes of interest (VOIs), containing skin and fat tissues at the forehead (VOI1) and the chin (VOI2) (Figure 4, left column). Finally, we calculated the percentage reduction in the area bounded under the tissue exposure curve when dressings have been applied with respect to the no‐dressing case, separately for the effective stress and SED data, and per each aforementioned anatomical VOI.
FIGURE 4.
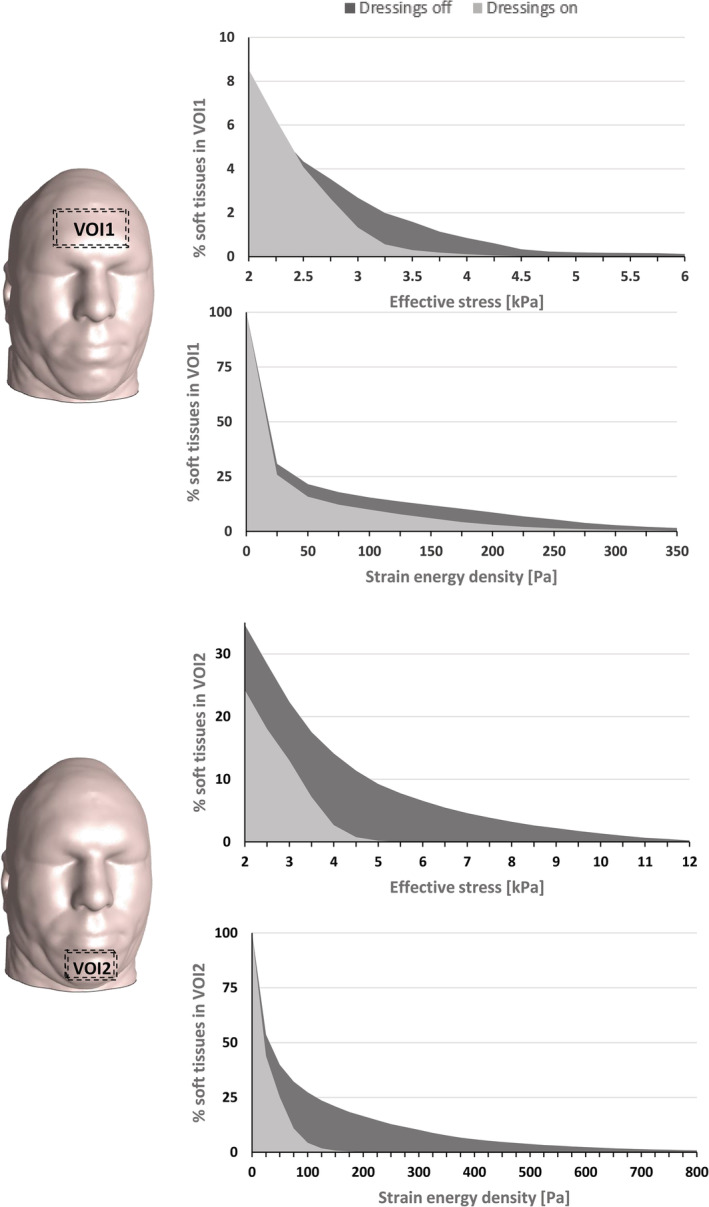
Cumulative percentages of soft tissue exposures to loading in volumes of interests (VOIs, marked as dashed boxes on the head model) containing facial skin and subcutaneous fat at the forehead (VOI1) and the chin (VOI2). The upper and lower plots per each VOI depict exposures to effective stresses and strain energy densities, respectively
3. RESULTS
The effective stress and SED distributions that developed on the facial skin with the applied dressings and without dressings are shown in Figure 2. Without dressings, effective skin stress concentrations peaked at 7.3 kPa and 28.9 kPa at the forehead and chin, respectively. The patterns of these stress concentrations on the skin were elongated at the forehead (along the ear‐to‐ear direction) and diffused at the chin (Figure 2; left column). Both stress concentrations reduced substantially in peak magnitudes (by 33% and 80% for the forehead and chin locations, respectively) and in their VOIs when the dressings were applied prophylactically (Figure 2; right column). Analysis of the SED distributions in the skin and subcutaneous fat tissues revealed similar concentration patterns, but with more spread of the forehead SED concentration in fat than in skin (as fat tissue is less stiff and thereby deforms more). Peak SED values without dressings were 1.8 and 5.1 kPa at the forehead and chin, respectively (Figure 3; left column). Again, the peak SED magnitudes decreased considerably with the dressings as tissue protectants (on skin: by 61% and 91%; in fat: by 35%, and 84% for the forehead and chin in each tissue, respectively; Figure 3, right column).
FIGURE 2.
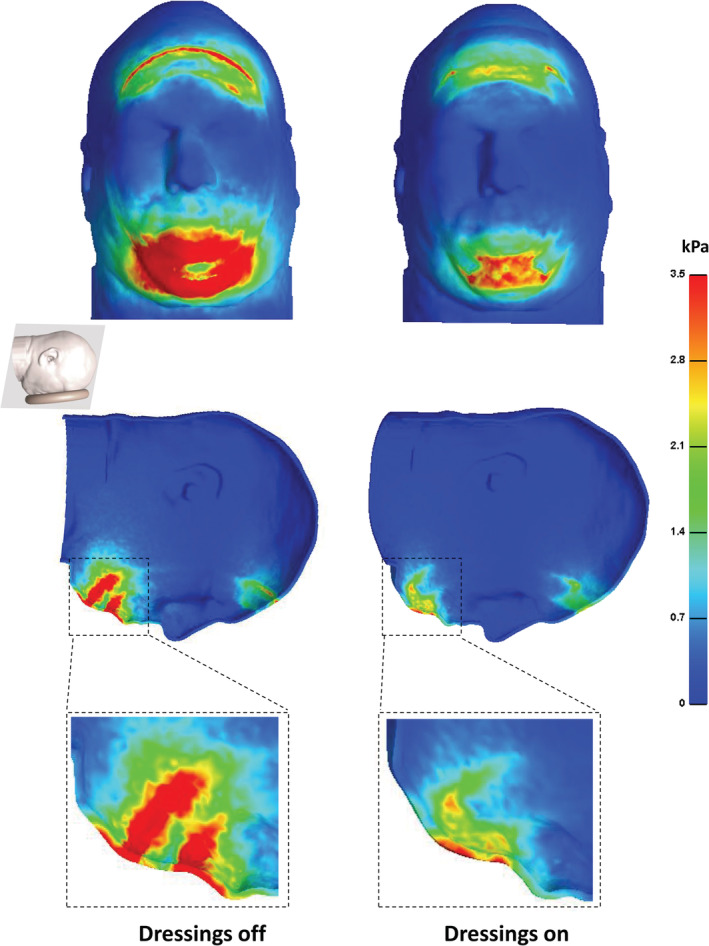
The effective stress distributions developed on the facial skin with applied Mepilex Border Flex (Mölnlycke Health Care, Gothenburg, Sweden) dressings (right frame) vs without dressings (left frame). The top and bottom frames show inferior and side views of the head in the prone position (with zooms of stress concentrations at the chin region), respectively
FIGURE 3.
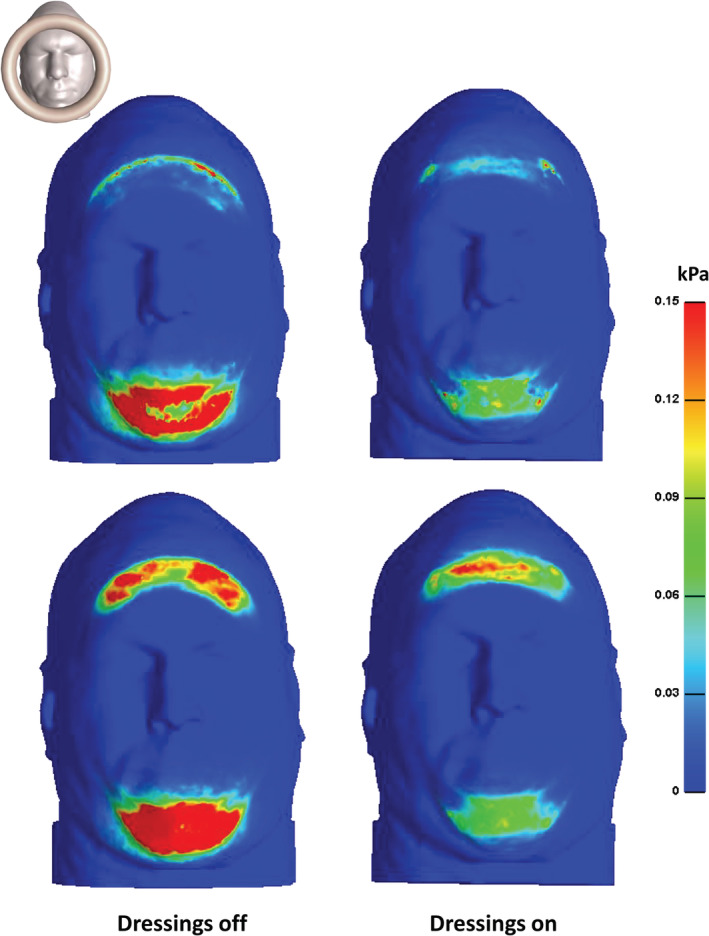
Strain energy density distributions on facial skin (top frames) and in subcutaneous fat (lower frames) from an inferior view of the head in the prone position, with applied Mepilex Border Flex (Mölnlycke Health Care, Gothenburg, Sweden) dressings (right column) vs without dressings (left column)
Analysis of the tissue loading exposure curves (Figure 4) was consistent with the above results and specifically indicated that with respect to the forehead (VOI1), application of a dressing resulted in 52% and 71% reductions in soft tissues exposures to effective stresses and SEDs, respectively (Figure 4A). Likewise, with regard to the chin (VOI2), a dressing lowered the volumetric soft tissue exposures to effective stresses and SED by 78% and 92%, respectively (Figure 4B).
4. DISCUSSION
Prone positioning is used in many invasive procedures in different surgical fields. 6 , 7 , 17 , 20 , 28 Despite the large variety of operation types requiring prone positioning, there is no published research concerning the biomechanical efficacy of PUP technologies for protecting prone surgical patients. During the present COVID‐19 pandemic, the use of prone positioning has expanded sharply, in ICUs, as those patients developing ARDS and who are mechanically ventilated are typically placed prone for sessions of approximately 16 hours or more and up to 24 hours, to improve their lung mechanics and tissue oxygenation. 12 , 13 , 14 New clinical guidelines have been released in this regard, recommending to apply pressure redistributing devices and prophylactic dressings to protect these COVID‐19 patients from facial PUs. 15 , 16 , 43 Nevertheless, there is scarce bioengineering research specific to PUP in prone positioning to support these urgently issued guidelines, particularly with regard to the biomechanical efficacy of prophylactic dressings in protecting prone patients from facial PUs. The present work was, therefore, conducted to address these critical and unmet needs.
In the above context, we evaluated facial tissue exposures to sustained head weight forces during a prone surgical or intensive care position, with or without prophylactic dressings applied at the forehead and chin. For this purpose, we developed two configurations of a computational, 3D anatomically realistic validated adult head model, which is rested prone, with or without the protective dressings, and used the FE method to analyse the skin and subcutaneous fat tissue loading states in each such configuration. Consistent with our published work on the efficacy of soft silicone‐foam multi‐layered prophylactic dressings in alleviating skin and deeper tissue loads, 47 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 64 , 65 we found that the presently studied dressings have a remarkable protective effect on facial tissues (Figures 2 and 3). We attribute this protective efficacy to multiple factors that are all related to the engineering design of the specific dressings that were studied here. First, the dressings are soft and thick to the optimal extent that provides localised cushioning at the primary support contact areas of the prone head, that is, the forehead and chin. Second, the multi‐layered alternating stiffness structure of these dressings is effective in absorbing shear loads within the dressing, as demonstrated in our published work with regard to other body parts, for example, the sacrum and heels. 53 , 54 , 55 , 56 , 57 , 58 , 59 , 64 , 65 Third, the considerable flexibility of the dressing structure (achieved through the repeated Y‐shaped cut patterns embedded in its retention and spreading layers) appears to add to its capability of alleviating the bodyweight loads, as we have demonstrated before for sacral multi‐layered prophylactic dressings tested against simple and conventional dressing designs (Figures 2 and 3). 54 , 56 , 58 , 59
In clinical practice, multi‐layered silicone‐foam prophylactic dressings have become a widely accepted adjunct PUP method in patients considered to be at‐risk. Clinical trials conducted with these dressings in patients in the supine position have demonstrated positive outcomes. 45 , 46 While there are limited experimental studies in the form of clinical trials for the use of these dressings in patients in the prone position, the biomechanical evidence presented here supports the use of these dressings to reduce facial PUs during prone positioning. The possibility of integrating research into clinical care can take considerable time initially to obtain the necessary ethical approvals for the research, and later to fully train the study team before initiating the project. Public health emergencies, such as the COVID‐19 pandemic, create an urgent need to prevent negative outcomes, including PUs, and therefore, to use materials that are currently available and techniques we already know. The use of soft silicone‐foam multi‐layered prophylactic dressing is a practical solution in clinical care, and the present computational modelling work strongly supports this approach.
The above dressings have been recommended for and applied to areas at‐risk of PUs in COVID‐19 prone patients. 15 , 43 Recent publications concerning prone patients suggest that soft silicone‐foam multi‐layered prophylactic dressings are generally well‐tolerated and demonstrate adjunct efficacy in PUP. 66 , 67 These dressings minimise shearing of the skin when the patient is placed in the prone position and also wick away moisture and allow perspiration to evaporate and not remain on the skin, which further contributes to the reduction of the frictional forces acting on the skin. 68 , 69 , 70 , 71
Considerations for use of prophylactic dressings on the facial area comprise the dressing sizes, the applied skin assessment protocol, and the frequency of dressing changes. The dressing sizes used for COVID‐19 prone patients clearly depend on the head dimensions of the individual, but coauthor MBJ who treated a substantial number of COVID‐19 patients noted that the dressing types studied here were suitably sized for protecting the face of COVID‐19 patients at her medical facility. Pulling back the dressings and inspecting the skin beneath them on a daily basis is recommended. The recommended frequency of dressing changes is given in the manufacturer's instructions. Lastly, these dressings should be used in conjunction with other comprehensive PUP strategies, such as careful positioning and small micro‐shifts to the head and chin every 2 hours to offload pressures. 15
The analysis of SED distributions in the skin and subcutaneous fat tissues revealed similar concentration patterns, but with more intensity and spread of the forehead SED concentrations in fat than in skin, suggesting greater risk for a forehead DTI than direct skin damage for prone positioning (Figure 3). Indeed, clinical reports describe forehead injuries associated with prone positioning as DTIs rather than as skin lesions. 7 , 35 , 36 , 37 , 38 , 39 The involvement of the DTI pathway calls for applying a more holistic approach in protecting tissues so that the prophylactic measures target both the superficial and deeper facial soft tissue structures. The latter, deep tissue protection can be achieved, for example, by using the fluidised positioner technology, which has been demonstrated to have high biomechanical efficacy in protecting scalp tissues, and is, therefore, likely to also be effective in facial protection against PUs. 44 , 49
Limitations are inevitable in any modelling work and should be discussed here for completeness. An important assumption is that the anatomy and biomechanical properties of tissues considered in the modelling represent healthy conditions, and do not account for aged, diabetic, or abnormally fragile skin. While prophylactic dressings will not necessarily induce the same biomechanical conditions in tissues of different individuals, they are expected, based on the present work, to substantially lower the patient‐specific tissue load levels with respect to a no‐dressing‐protection state. In other words, the application of dressings on the forehead and chin of prone patients will always provide facial tissue protection at the sites of application, but not the same level of protection for all patients. The individual level of protection would depend not only on the skin conditions but also on the subdermal tissue quality, the shapes of the skull surfaces and the weight of the head of the person, as well as on the functions of the body system that are relevant to PU aetiology, particularly the inflammatory and cardiovascular systems, as reviewed in the literature. 71 , 72 , 73 , 74
5. CONCLUSIONS
To conclude, in this study, we found that applying prophylactic dressings of the type studied here to the forehead and chin remarkably alleviates the facial soft tissue loads at these sites when the head is prone, by more than 50% (at both sites) with respect to the no‐dressing case. Accordingly, this study provides, for the first time, solid biomechanical evidence to support the practice of nurses in applying multi‐layered silicone‐foam dressings to protect the face of patients undergoing prone surgeries or ICU treatments. The present work is more relevant than ever, given the exponential rise in the numbers of mechanically ventilated ARDS COVID‐19 patients added to the global healthcare system with the current pandemic. 12 , 13 , 14 , 15 , 16 , 43 A next relevant research step would be to determine the microclimate under prophylactic dressings in a prone position, as evaluated in our previous work, 75 , 76 particularly since fever is a common symptom of COVID‐19. This question can be addressed by means of the multi‐physics computational modelling approaches recently developed and reported by our research group. 47 , 77 , 78 Finally, more evidence in the form of bioengineering modelling work and clinical research is needed to guide practice and to inform the use of soft silicone‐foam multi‐layered prophylactic dressings to not only prevent facial PUs but also for using dressings at other pressure points in a prone position.
ACKNOWLEDGEMENT
This work was supported by an educational grant from Mölnlycke Health Care (Gothenburg, Sweden).
Peko L, Barakat‐Johnson M, Gefen A. Protecting prone positioned patients from facial pressure ulcers using prophylactic dressings: A timely biomechanical analysis in the context of the COVID‐19 pandemic. Int Wound J. 2020;17:1595–1606. 10.1111/iwj.13435
Funding information Mölnlycke Health Care
REFERENCES
- 1. European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Ulcer Advisory Panel (NPUAP) and the Pan‐Pacific Alliance (PPA) , International Pressure Ulcer Guidelines. Westford, MA, USA: EPUAP‐NPIAP‐PPPIA; 2019. www.epuap.org/pu-guidelines/#2014guidelines&qrg. [Google Scholar]
- 2. Gefen A. The biomechanics of sitting‐acquired pressure ulcers in patients with spinal cord injury or lesions. Int Wound J. 2007;4:222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manorama A, Meyer R, Wiseman R, Bush TR. Quantifying the effects of external shear loads on arterial and venous blood flow: implications for pressure ulcer development. Clin Biomech. 2013;28(5):574‐578. [DOI] [PubMed] [Google Scholar]
- 4. Shoemake S, Stoessel K. The clinical issue: pressure ulcers in the surgical patients. Halyard Knowledge Network. 2015;1:1–11. https://www.halyardhealth.com/media/1513/c14222-clinical-issue-one-pressure-ulcers.pdf. [Google Scholar]
- 5. Scarlatti KC, Michel JL, Gamba MA, de Gutiérrez MG. Pressure ulcers in surgery patients: incidence and associated factors. Rev Esc Enferm USP. 2011;45(6):1372‐1379. [DOI] [PubMed] [Google Scholar]
- 6. Kwee MM, Ho YH, Rozen WM. The prone position during surgery and its complications: a systematic review and evidence‐based guidelines. Int Surg. 2015;100(2):292‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimsey DB. A change in focus: shifting from treatment to prevention of perioperative pressure injuries. AORN J. 2019;110(4):379‐393. [DOI] [PubMed] [Google Scholar]
- 8. Grisell M, Place HM. Face tissue pressure in prone positioning: a comparison of three face pillows while in the prone position for spinal surgery. Spine. 2008;33(26):2938‐2941. [DOI] [PubMed] [Google Scholar]
- 9. Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Res Nurs Health. 1994;17:333‐339. [DOI] [PubMed] [Google Scholar]
- 10. Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs. 1999;26:130‐136. [DOI] [PubMed] [Google Scholar]
- 11. Joshua LL, Syed AA, Loic ST. Retrospective review of predisposing factors for intraoperative pressure ulcer development. J Clin Anesthesia. 2014;26:368‐374. [DOI] [PubMed] [Google Scholar]
- 12. Pan C, Chen L, Lu C, et al. Lung Recruitability in SARS‐CoV‐2 associated acute respiratory distress syndrome: a single‐center, observational study. Am J Respir Crit Care Med. 2020;201:1294‐1297. 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of ciritially ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scholten EL, Beitler JR, Prisk K, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Pressure Injury Advisory Panel (NPIAP) . Pressure Injury Prevention: PIP Tips for Prone Positioning. Westford MA, USA: NPIAP; 2020. https://www.nestlemedicalhub.com/sites/site.prod.nestlemedicalhub.com/files/2020-04/npiap_pip_tips_proning_202.pdf. [Google Scholar]
- 16. Bamford P, Bentley A, Dean J, David Whitmore D, Wilson‐Baig N. Guidance for Prone Positioning of the Conscious COVID Patient. Int Care Soc. 2020;1–6. https://emcrit.org/wp-content/uploads/2020/04/2020-04-12-Guidance-for-conscious-proning.pdf. [Google Scholar]
- 17. DePasse JM, Palumbo MA, Haque M, Eberson CP, Daniels AH. Complications associated with prone positioning in elective spinal surgery. World J Orthop. 2015;6(3):351‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabara Y, Tachibana‐Iimori R, Yamamoto M, et al. Hypotension associated with prone body position: a possible overlooked postural hypotension. Hypertens Res. 2005;28:741‐746. [DOI] [PubMed] [Google Scholar]
- 19. Dharmavaram S, Jellish WS, Nockels RP, et al. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine J. 2006;31:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 20. Schonauer C, Bocchetti A, Barbagallo G, Albanese V, Moraci A. Positioning on surgical table. Eur Spine J. 2004;13(suppl 1):S50‐S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slocum HC, O'neal KC, Allen CR. Neurovascular complications from malposition on the operating table. Surg Gynecol Obstet. 1948;86:729‐734. [PubMed] [Google Scholar]
- 22. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5:100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singer MS, Salim S. Bilateral acute angle‐closure glaucoma as a complication of facedown spine surgery. Spine J. 2010;10:7‐9. [DOI] [PubMed] [Google Scholar]
- 24. Tong CK, Chen JC, Cochrane DD. Spinal cord infarction remote from maximal compression in a patient with Morquio syndrome. J Neurosurg Pediatr. 2012;9:608‐612. [DOI] [PubMed] [Google Scholar]
- 25. Chen SH, Hui YL, Yu CM, Niu CC, Lui PW. Paraplegia by acute cervical disc protrusion after lumbar spine surgery. Chang Gung Med J. 2005;28:254‐257. [PubMed] [Google Scholar]
- 26. Uribe JS, Kolla J, Omar H, et al. Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13:552‐558. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of positioninduced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437‐444. [DOI] [PubMed] [Google Scholar]
- 28. Hartley J. Patient Positioning During Anesthesia. General Anesthesia, Tutorial 311. London, UK: The World Federation of Societies of Anaesthesiologists; 2015. https://www.wfsahq.org/components/com_virtual_library/media/a5da94469c304896052227a7b047c785-311-Patient-positioning-during-anaesthesia.pdf. [Google Scholar]
- 29. Koreckij J, Price N, Schwend RM. Vectored cranial‐cervical traction limits facial contact pressure from prone positioning during posterior spinal deformity surgery. Spine (Phila Pa 1976). 2011;36(15):E993‐E997. [DOI] [PubMed] [Google Scholar]
- 30. Dudek NL, Buenger UR, Trudel G. Bilateral anterior superior iliac spine pressure ulcers: a case report. Arch Phys Med Rehabil. 2002;83:1459‐1461. [DOI] [PubMed] [Google Scholar]
- 31. Goodwin CR, Recinos PF, Omeis I, et al. Prevention of facial pressure ulcers using the Mayfield clamp for sacral tumor resection. J Neurosurg Spine. 2011;14:85‐87. [DOI] [PubMed] [Google Scholar]
- 32. Spector WD, Limcangco R, Owens PL, Steiner CA. Marginal hospital cost of surgery‐related hospital‐acquired pressure ulcers. Med Care. 2016;54(9):845‐851. [DOI] [PubMed] [Google Scholar]
- 33. Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy Wound Manage. 1999;45:34‐44. [PubMed] [Google Scholar]
- 34. Chalian AA, Kagan SH. Backside first in head and neck surgery: preventing pressure ulcers in extended length surgeries. Head Neck. 2001;23:25‐28. [PubMed] [Google Scholar]
- 35. Alderden J, Whitney JD, Taylor SM, Zaratkiewicz S. Risk profile characteristics associated with outcomes of hospital‐acquired pressure ulcers: a retrospective review. Crit Care Nurse. 2011;31(4):30‐43. [DOI] [PubMed] [Google Scholar]
- 36. Shamshery C, Haldar R, Srivastava A, Kaushal A, Srivastava S, Singh PK. An unusual cause of unilateral facial injuries caused by horseshoe headrest during prone positional craniovertebral junction surgery. J Craniovertebr Junction Spine. 2016;7(1):62‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walton‐Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J. 2009;89(3):538‐548. [DOI] [PubMed] [Google Scholar]
- 38. Kalowes P, Messina V, Li M. Five‐layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care. 2016;25(6):e108‐e119. [DOI] [PubMed] [Google Scholar]
- 39. Nazerali RS, Song KR, Wong MS. Facial pressure ulcer following prone positioning. J Plast Reconstr Aesthet Surg. 2010;63(4):e413‐e414. [DOI] [PubMed] [Google Scholar]
- 40. Gefen A, Creehan S, Black J. Critical biomechanical and clinical insights concerning tissue protection when positioning patients in the operating room: A scoping review. Int Wound J. 2020; accepted for publication. 10.1111/iwj.13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henderson WR, Griesdale DE, Dominelli P, Ronco JJ. Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can Respir J. 2014;21(4):213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JT, Kim HJ, Ahn W, et al. Head rotation, flexion, and extension alter endotracheal tube position in adults and children. Can J Anaesth. 2009;56:751‐756. [DOI] [PubMed] [Google Scholar]
- 43. Guideline for positioning the patient . Guidelines for Perioperative Practice. Denver, CO: AORN; 2019:637‐714. [Google Scholar]
- 44. Katzengold R, Gefen A. What makes a good head positioner for preventing occipital pressure ulcers. Int Wound J. 2018;15(2):243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi‐layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12(3):302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santamaria N, Gerdtz M, Liu W, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: border II trial. J Wound Care. 2015;24(8):340‐345. [DOI] [PubMed] [Google Scholar]
- 47. Peko Cohen L, Ovadia‐Blechman Z, Hoffer O, Gefen A. Dressings cut to shape alleviate facial tissue loads while using an oxygen mask. Int Wound J. 2019;16(3):813‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Visible Human Project Gallery . Visible Human Project (VHP) of the U.S. National Library of Medicine. Bethesda, MD: U.S. National Library of Medicine; 1994. [Google Scholar]
- 49. Katzengold R, Gefen A. Modelling an adult human head on a donut‐shaped gel head support for pressure ulcer prevention. Int Wound J. 2019;16:1398‐1407. 10.1111/iwj.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Friedman R, Haimy A, Gefen A, Epstein Y. Three‐dimensional biomimetic head model as a platform for thermal testing of protective goggles for prevention of eye injuries. Clin Biomech. 2018;64:35–41. 10.1016/j.clinbiomech.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 51. Simpleware® Ltd . ScanIP, +FE, +NURBSand +CADReference Guide ver 5.1. Mountain View CA, USA: Synopsys Inc.; 2012. http://www.simpleware.com/software/. [Google Scholar]
- 52. Goldstein JP. The effect of motorcycle helmet use on the probability of fatality and the severity of head and neck injuries a latent variable frame‐work. Eval Rev. 1986;10(3):355‐375. [Google Scholar]
- 53. Gefen A, Alves P, Creehan S, Call E, Santamaria N. Computer modeling of prophylactic dressings: An indispensable guide for healthcare professionals. Adv Skin Wound Care. 2019;32(7S suppl 1):S4–S13. [DOI] [PubMed] [Google Scholar]
- 54. Levy A, Schwartz D, Gefen A. The contribution of a directional preference of stiffness to the efficacy of prophylactic sacral dressings in protecting healthy and diabetic tissues from pressure injury: computational modelling studies. Int Wound J. 2017;14(6):1370‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levy A, Frank MB, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 56. Levy A, Gefen A. Assessment of the biomechanical effects of prophylactic sacral dressings on tissue loads: a computational modeling analysis. Ostomy Wound Manage. 2017;63:48‐55. [PubMed] [Google Scholar]
- 57. Levy A, Gefen A. Computer modeling studies to assess whether a prophylactic dressing reduces the risk for deep tissue injury in the heels of supine patients with diabetes. Ostomy Wound Manage. 2016;62:42‐52. [PubMed] [Google Scholar]
- 58. Schwartz D, Levy A, Gefen A. A computer modeling study to assess the durability of prophylactic dressings subjected to moisture in biomechanical pressure injury prevention. Ostomy Wound Manage. 2018;64(7):18‐26. [PubMed] [Google Scholar]
- 59. Schwartz D, Gefen A. The biomechanical protective effects of a treatment dressing on the soft tissues surrounding a non‐offloaded sacral pressure ulcer. Int Wound J. 2019;16(3):684‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Linder‐Ganz E, Gefen A. Stress analyses coupled with damage laws to determine biomechanical risk factors for deep tissue injury during sitting. J Biomech Eng. 2009;131(1):011003. 10.1115/1.3005195. [DOI] [PubMed] [Google Scholar]
- 61. Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for bio‐mechanics. J Biomech Eng. 2012;134(1):5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. FEBio , Finite element for biomechanics, theory manual ver. 1.5. Salt Lake City UT, USA: The University of Utah; 2012. http://mrl.sci.utah.edu/software/febio. [Google Scholar]
- 63. Peko Cohen L, Gefen A. Deep tissue loads in the seated buttocks on an off‐loading wheelchair cushion versus air‐cell‐based and foam cushions: finite element studies. Int Wound J. 2017;14:1327‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peko Cohen L, Levy A, Shabshin N, Neeman Z, Gefen A. Sacral soft tissue deformations when using a prophylactic multilayer dressing and positioning system: MRI studies. J Wound Ostomy Continence Nurs. 2018;45(5):432‐437. [DOI] [PubMed] [Google Scholar]
- 65. Gefen A, Kottner J, Santamaria N. Clinical and biomechanical perspectives on pressure injury prevention research: the case of prophylactic dressings. Clin Biomech. 2016;38:29‐34. [DOI] [PubMed] [Google Scholar]
- 66. Kim RS, Mullins K. Preventing facial pressure ulcers in acute respiratory distress syndrome (ARDS). J Wound Ostomy Continence Nurs. 2016;43(4):427‐429. [DOI] [PubMed] [Google Scholar]
- 67. Yoshimura M, Ohura N, Tanaka J, et al. Soft silicone foam dressing is more effective than polyurethane film dressing for preventing intraoperatively acquired pressure ulcers in spinal surgery patients: the border operating room spinal surgery (BOSS) trial in Japan. Int Wound J. 2018;15(2):188‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Black J, Alves P, Brindle CT, et al. Use of wound dressings to enhance prevention of pressure ulcers caused by medical devices. Int Wound J. 2015;12(3):322‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwartz D, Magen YK, Levy A, Gefen A. Effects of humidity on skin friction against medical textiles as related to prevention of pressure injuries. Int Wound J. 2018;15(6):866‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gefen A, Ousey K. Update to device‐related pressure ulcers: SECURE prevention. COVID‐19, face masks and skin damage. J Wound Care. 2020;29(5):245‐259. [DOI] [PubMed] [Google Scholar]
- 71. Gefen A, Alves P, Ciprandi G, et al. Device‐related pressure ulcers: SECURE prevention. J Wound Care. 2020;29(suppl 2a):S1–S52. [DOI] [PubMed] [Google Scholar]
- 72. Gefen A. The future of pressure ulcer prevention is here: detecting and targeting inflammation early. EWMA J. 2018;19(2):7‐13. [Google Scholar]
- 73. Gefen A. How medical engineering has changed our understanding of chronic wounds and future prospects. Med Eng Phys. 2019;72:13‐18. [DOI] [PubMed] [Google Scholar]
- 74. Gefen A, Brienza D, Edsberg L, et al. The etiology of pressure injuries. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP) and the Pan Pacific Pressure Injury Alliance (PPPIA). 3rd ed. Westford MA, USA: EPUAP‐NPIAP‐PPPIA; 2019. [Google Scholar]
- 75. Amrani G, Peko L, Hoffer O, Ovadia‐Blechman Z, Gefen A. The microclimate under dressings applied to intact weight‐bearing skin: infrared thermography studies. Clin Biomec. 2020;75:104994. 10.1016/j.clinbiomech.2020.104994. [DOI] [PubMed] [Google Scholar]
- 76. Gefen A, Cohen LP, Amrani G, Hoffer O, Ovadia‐Blechman Z. The roles of infrared thermography in pressure ulcer research with focus on skin microclimate induced by medical devices and prophylactic dressings. Wounds Int. 2019;10(1):8‐15. [Google Scholar]
- 77. Zeevi T, Levy A, Brauner N, Gefen A. Effects of ambient conditions on the risk of pressure injuries in bedridden patients – multi‐physics modelling of microclimate. Int Wound J. 2017;15(3):402‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schwartz D, Gefen A. An integrated experimental‐computational study of the microclimate under dressings applied to intact weight‐bearing skin. Int Wound J. 2020;17:562‐577. 10.1111/iwj.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Linder‐Ganz E, Shabahin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub‐dermal tissues during sitting: a combined experimental‐MRI and finite element approach. J Biomech. 2007;40(7):1443‐1454. [DOI] [PubMed] [Google Scholar]
- 80. Sopher R, Nixon J, Gorecki C, Gefen A. Exposure to internal muscle tissue loads under the ischial tuberosities during sitting is elevated at abnormally high or low body mass indices. J Biomech. 2010;43(2):280‐286. [DOI] [PubMed] [Google Scholar]
- 81. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129(6):924‐930. [DOI] [PubMed] [Google Scholar]
- 82. Moore DF, Jérusalem A, Nyein M, Noels L, Jaffee MS, Radovitzky RA. Computational biology‐ modeling of primary blast effects on the central nervous system. Neuroimage. 2009;47(suppl 2):T10‐T20. [DOI] [PubMed] [Google Scholar]


