In view of numerous recent reports describing increased risks of thrombosis in coronavirus disease 2019 (COVID‐19) amongst certain ethnicities in which co‐morbidities are more prevalent, besides the identified risk factors, procoagulant plasma extracellular vesicles have not been considered. Several comorbidities predict mortality in patients with COVID‐19, some of which are more prevalent in Black, Asian and Minority Ethnic (BAME) groups. In a meta‐analysis of seven studies (1,576 infected patients) the co‐morbidities included hypertension (21·1%), diabetes (9·7%), cardiovascular disease (CVD), (8·4%) and respiratory system disease (1·5%). 1 Of the critically ill COVID‐19 patients with these co‐morbidities and an associated hyperinflammatory state admitted to intensive care units (ICUs), up to 31% suffered thrombotic episodes (even with thromboprophylaxis), in particular, venous thromboembolisms (VTE), 2 one of the important sequelae of COVID‐19.
Extracellular vesicles (EVs) are nano‐sized, membrane‐bound vesicles released from cells that carry nucleic acids and proteins, and which mediate intercellular communication. In COVID‐19‐associated co‐morbidities, including diabetes, CVD and risk factors such as hypertension, elevated angiotensin II (Ang II) and obesity, levels of circulating EVs are raised. 3 , 4 , 5 , 6 Increased EVs, especially from injured or TNF‐α‐stimulated endothelial cells (EC) carrying tissue factor (TF+), or from platelets exposing phosphatidylserine (PS+), are known to be procoagulant and to cause VTE. 7 Levels of EVs also correlate with von Willebrand Factor (vWF), a marker of EC damage and dysfunction. 8 This endothelial injury is due to Ang II‐mediated superoxide damage and hypoxia‐mediated oxidative stress as well as severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) binding to angiotensin‐converting enzyme 2 (ACE2) on ECs and ensuing complement‐mediated inflammation 9 (Fig 1A,B). Previously, endothelial cell‐derived medium EV (EC‐mEV) levels were found to be associated with stroke. 10 More recently, CD31+ and CD144+ EC‐derived mEVs, phenotypes reflecting apoptosis and structurally damaged endothelium, were found to relate to risk factors for CVD and, thus, increased the risk of VTE, especially raised triglycerides, hypertension and metabolic syndrome. 4
Fig 1.
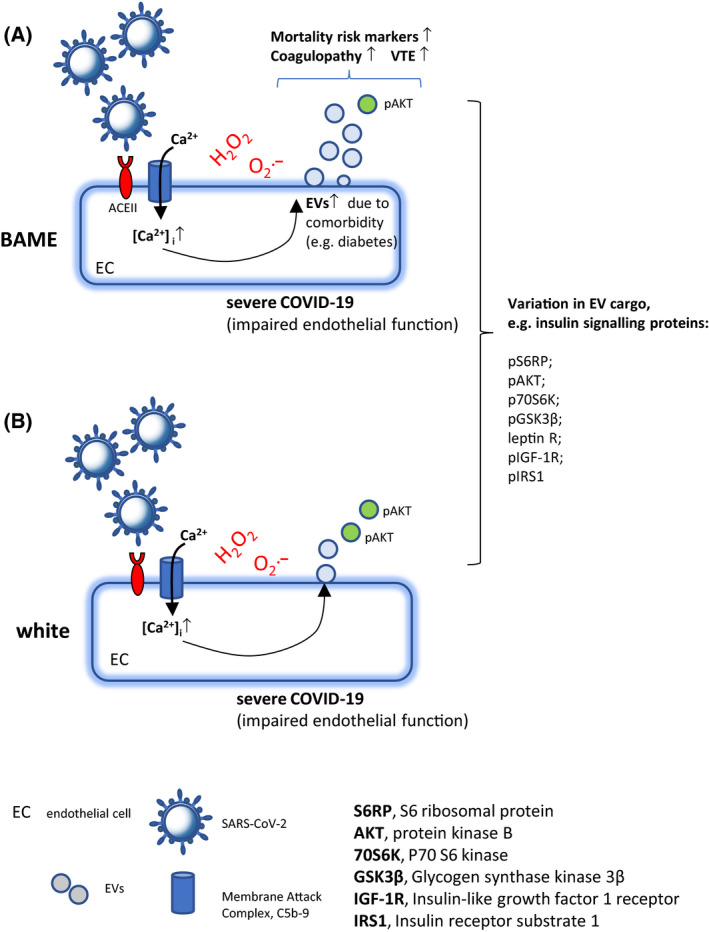
(A and B) Extracellular vesicles (EVs) and coagulopathy in COVID‐19 and a possible link with ethnicity. SARS‐CoV‐2 infection activates the complement and formation of membrane attack complex (MAC, C5b‐9). The resulting Ca2+ influx through deposited C5b‐9 and by the SARS‐CoV‐2 E‐protein Ca2+ channel from Endoplasmic reticulum‐Golgi intermediate compartment/endoplasmic reticulum (ERGIC/ER), leads to Ca2+‐mediated EV release. Circulating EVs carrying phosphorylated insulin signalling proteins and related to diabetes mellitus, an important comorbidity in COVID‐19, are associated with clinical mortality risk markers linked to ethnicity. (A) In severe COVID‐19, in particular in ethnicities with increased comorbidities such as diabetes, and associated circulating EV levels, EVs with their known role in coagulopathy could play an important role in COVID‐19‐related deaths through VTE. BAME. Black Asian and Minority Ethnic groups.
Could it therefore, be that raised circulating EVs with modulated cargo in certain comorbidity groups, in addition to infection with SARS‐CoV‐2, renders COVID‐19 patients particularly susceptible to VTE? If so, EVs could be used as biomarkers in COVID‐19. A TF+‐EV procoagulant activity assay, for example, based on fibrin formation, previously used in monitoring the risk of VTE in cancer patients, should be tried in COVID‐19.
It was recently reported that higher rates of death from COVID‐19 in the UK were found in BAME patients. 11 This study used the Standardized Mortality Ratio (SMR) to calculate the risk of death (adjusted for age and region), for BAME patients compared to the general population. The adjusted ethnic‐specific SMRs were as follows: 3·29 (Pakistani), 3·24 (Black African), 2·41 (Bangladeshi), 2·21 (Black Caribbean) and 1·7 (Indian). In a UK study by Valabhji et al. (in preprint: https://www.england.nhs.uk/wp‐content/uploads/2020/05/Valabhji‐COVID‐19‐and‐Diabetes‐Paper‐2‐Full‐Manuscript.pdf), assessing the risk people with diabetes face of dying of COVID‐19, 418 COVID‐19‐related deaths from 265 090 people with Type 1 diabetes (T1D) and 9,377 deaths from 2 889 210 people with T2D were analysed. It was found that there was a higher mortality risk of COVID‐19‐related death for people of Asian or Black ethnicities with diabetes. Specifically, for T1D the adjusted hazard ratio, was 1·79 and 1·68 for the Asian and Black groups respectively, compared to the White group. For T2D the hazard ratio was 1·09 for Asian and 1·63 for people of Black ethnicity, compared to the White group. In the International Severe Acute Respiratory and Emerging Infections Consortium/UK Clinical Characterisation Protocol (ISARIC/CCP‐UK) study, looking at 34 968 patients admitted to hospitals in England, Scotland and Wales, South Asians with COVID‐19 were 20% more likely to die than Whites. 12 Compared to the White group, South Asians had the highest hazard ratio of death (1·19) followed by Black patients (1·05) and around 40% of the South Asian and Black patients had T1D or T2D. This pre‐existing co‐morbidity accounted particularly for the excess mortality observed amongst the South Asians.
If plasma EV levels are raised, and cargo modified in co‐morbidities associated with COVID‐19, such as diabetes, account for excess deaths in BAME patients, could EVs be playing a role and might there be a connection to ethnicity? In the United States, African Americans (AA) show a disproportionately high co‐mborbidity‐associated death rate from COVID‐19, compared to White Americans (WA). 13 Interestingly, in a study looking firstly at the concentration and mean/modal sizes of circulating plasma EVs in AA and WA, regardless of gender, no ethnic difference was found. 14 However, assessing EV protein content involved in insulin signalling, the study found lower levels of phospho‐AKT (pAKT) in AAs compared to WAs, which is associated with impaired insulin signalling, insulin resistance and an increased risk of VTE. Importantly, considering phosphorylated insulin signalling proteins, shown previously by the same study to be in EVs and related to diabetes mellitus, 2 , 3 the association of these EV proteins with clinical mortality risk markers, such as serum creatinine, lactate dehydrogenase, homeostatic model assessment of β‐cell function, serum alkaline phosphatase and pulse pressure, did seem to depend on ethnicity.
The connection between EV cargo, mortality markers and race may be of considerable importance because of the current knowledge gap in susceptibility for COVID‐19 amongst BAME patients. We know that EVs play a role in T2D 3 and CVD, 4 and may thus constitute important risk factors leading to VTE in COVID‐19 patients. It is already known that proteins such as vWF, which contributing to VTE, show an association with ethnicity. 15 However, given that certain EV proteins and their relationship with clinical mortality markers may also vary according to ethnicity (see Fig 1A,B), and contribute to clinical outcomes, now might be an opportune time to begin to use EV carrying molecules as markers of risk of mortality in COVID‐19.
Acknowledgements
The author confirms being the sole author of this letter.
References
- 1. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67:2377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amabile N, Cheng S, Renard JM, Larson MG, Ghorbani A, McCabe E, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J. 2014;35:2972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003;41(2):211–7. [DOI] [PubMed] [Google Scholar]
- 6. Goichot B, Grunebaum L, Desprez D, Vinzio S, Meyer L, Schlienger JL, et al. Circulating procoagulant microparticles in obesity. Diabetes Metabolism. 2006;32:82–5. [DOI] [PubMed] [Google Scholar]
- 7. Zarà M, Guidetti GF, Camera M, Canobbio I, Amadio P, Torti M. Biology and role of extracellular vesicles (evs) in the pathogenesis of thrombosis. Int J Mol Sci. 2019;20(11):2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS‐CoV‐2 infection. Crit Care. 2020;24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–302. [DOI] [PubMed] [Google Scholar]
- 11. Aldridge RW, Lewer D, Katikireddi SV, Mathur R, Pathak N, Burns R, et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID‐19: indirect standardisation of NHS mortality data. Wellcome Open Res. 2020;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison E, Docherty AB, Barr BP, Buchan I, Carson G, Drake TM, et al. Ethnicity and outcomes from COVID‐19: the ISARIC CCP‐UK prospective observational cohort study of hospitalised patients. Lancet. 2020. 10.2139/ssrn.3618215 [DOI] [Google Scholar]
- 13. Holmes L Jr, Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, et al. Black‐white risk differentials in COVID‐19 (SARS‐COV2) transmission, mortality and case fatality in the united states: translational epidemiologic perspective and challenges. Int J Environ Res Public Health. 2020;17(12):4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noren Hooten N, McFarland MH, Freeman DW, Mode NA, Ezike N, Zonderman AB, et al. Association of extracellular vesicle protein cargo with race and clinical markers of mortality. Sci Rep. 2019;9:17582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost. 2006;4:2629–35. [DOI] [PubMed] [Google Scholar]


