Dear Editor,
We read the article by D'Alessandro et al with great interest and appreciate their efforts to evaluate the role of serum Krebs von den Lungen‐6 (KL‐6) as a prognostic biomarker of severe coronavirus disease 2019 (COVID‐19). 1 KL‐6 is a mucin‐like high‐molecular‐weight glycoprotein expressed in type II pneumocytes and respiratory bronchiolar epithelial cells in the healthy lung. 2 Elevated serum levels can predict the prognosis of various interstitial lung diseases (ILDs), including idiopathic pulmonary fibrosis (IPF), 3 and D'Alessandro et al 1 observed a significant increase in serum KL‐6 in critically ill patients with COVID‐19 who required mechanical ventilation or intensive care. Two COVID‐19‐related acute respiratory distress syndrome (CARDS) phenotypes exist: type L and type H, with preserved and low lung compliance (LC), respectively. 4 Recently, the Japan extracorporeal membrane oxygenation (ECMO)net for COVID‐19 working group reported that patients with type L COVID‐19 do not display increased serum KL‐6. 5 As biomarker detection in these studies 1 , 5 was performed at admission, changes in KL‐6 during COVID‐19 infection remain unexamined. We hypothesized that KL‐6 could help distinguish the L and H types of severe COVID‐19 during its clinical course. Here, we report different serum KL‐6 kinetics in two patients with severe COVID‐19. Serum KL‐6 was measured at admission (day 1), and on days 7, 14, and 21.
Case 1 was a 45‐year‐old male admitted due to COVID‐19 within 8 days of symptom onset (Figure 1). Chest computed tomography (CT) revealed consolidation in the subpleural areas of the bilateral upper lobe. Despite favipiravir, nafamostat, and tocilizumab treatment with supportive care, his ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) continued to deteriorate from 185 to 155. The patient was intubated on day 3 of admission, and ECMO was introduced on day 5 to prevent further lung impairment. The average LC before ECMO administration was 30.1 mL/cmH2O. After a 3‐day course of methylprednisolone (1 g/d), PaO2/FiO2 sharply improved. On day 14, ECMO was safely withdrawn, and the patient was extubated; his LC increased to 180 mL/cmH2O before extubation. Serum KL‐6 remained in the normal range throughout the clinical course (131‐363 U/mL).
Figure 1.
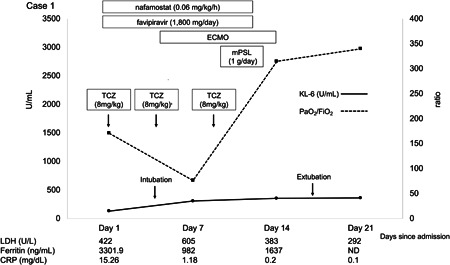
The clinical course of a 45‐year‐old patient with normal serum KL‐6 levels despite severe COVID‐19. CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; KL‐6, Krebs von den Lugen‐6; LDH, lactate dehydrogenase; mPSL, methylprednisolone; ND, no data; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; TCZ, tocilizumab
Case 2 was a 74‐year‐old male admitted due to COVID‐19, 8 days after symptom onset (Figure 2). He was treated with favipiravir and nafamostat. Chest CT revealed bilateral peripheral ground‐glass opacity. Despite treatment, the patient was intubated on day 3 of admission because of progressive acute respiratory failure (PaO2/FiO2 = 80). Although temporary PaO2/FiO2 improvement was observed after tocilizumab and methylprednisolone administration, respiratory parameters deteriorated afterward, and the patient died on day 33. Serum KL‐6 was increased at admission (673 U/mL) and increased steadily during the clinical course (to 2927 U/mL on day 21), while LC decreased from 54 to 14.63 mL/cmH2O. In both cases, the antiviral drug favipiravir was administered in the context of a clinical trial. The serine protease inhibitor nafamostat and the humanized anti‐human interleukin‐6 receptor antibody tocilizumab were administered off‐label.
Figure 2.
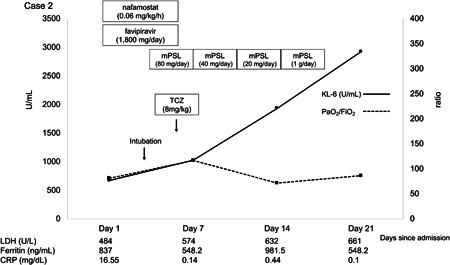
The clinical course of a 74‐year‐old patient with severe COVID‐19, showing a steady increase in serum KL‐6. CRP, C‐reactive protein; KL‐6, Krebs von den Lugen‐6; LDH, lactate dehydrogenase; mPSL, methylprednisolone; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; TCZ, tocilizumab
These cases reveal different kinetics of serum KL‐6 levels in two critically ill patients with COVID‐19. In ILDs, elevated KL‐6 reflects the progression of lung volume loss. 6 IPF prognosis is significantly worse when KL‐6 exceeds 1000 U/mL, 3 and the Italian COVID‐19 study produced consistent results. 1 The continuous increase in serum KL‐6 in case 2 was probably correlated with changes in lung elastance, as a follow‐up chest CT on day 31 revealed bronchiole dilation accompanied by extensive bilateral consolidation.
The results suggest that cases 1 and 2 were type L and H, respectively. The kinetics of KL‐6 in case 1 may be explained by the pathology of organizing pneumonia (OP), which produces radiographic patterns similar to COVID‐19. 7 KL‐6 levels can fluctuate in OP, 8 as damage to type II pneumocytes is typically influenced by underlying conditions. 9 Inflammatory debris in the alveolar space can affect lung compliance as well as gas exchange, but anti‐inflammatory agents are expected to cause rapid clearance unless severe type II pneumocyte impairment develops. Perhaps type L CARDS retains intact type II pneumocytes. As SARS‐Cov‐2 infects type II pneumocytes, 10 the case displaying no elevation in KL‐6 is intriguing.
The severity of the cytokine storm syndrome could affect the levels of serum KL‐6. At admission, the serum levels of lactate dehydrogenase, ferritin, and C‐reactive protein were 422 U/L, 3301.9 ng/mL, and 15.26 mg/dL, respectively in case 1, and 484 U/mL, 837 ng/mL, and 16.55 mg/dL, respectively in case 2. It is challenging to evaluate which case displayed a stronger cytokine storm based on these biomarkers. However, a significant difference in the ferritin levels of cases 1 and 2 suggests that case 1 may have had a more severe cytokine storm than case 2.
Presumably, age contributed to the poor outcome in case 2. In addition, subclinical ILDs might have affected the KL‐6 kinetics in this case, as KL‐6 was already elevated at admission. As an established treatment regimen was not yet available, the treatment protocols were left to the discretion of the doctors responsible for each case. The different protocols used may have affected the patients' outcomes and KL‐6 kinetics. Despite these limitations, these cases indicate that serum KL‐6 levels are a useful noninvasive tool to discern CARDS phenotypes and predict their prognosis. Further research is required to validate this hypothesis and determine what diverts the clinical course.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1. D'Alessandro M, Cameli P, Refini RM, et al. Serum KL‐6 concentrations as a novel biomarker of severe COVID‐19 [published online ahead of print May 29, 2020]. J Med Virol. 2020. 10.1002/jmv.26087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialyated carbohydrate antigen KL‐6. Chest. 1989;96:68‐73. [DOI] [PubMed] [Google Scholar]
- 3. Yokoyama A, Kondo K, Nakajima M, et al. Prognostic value of circulating KL‐6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164‐168. [DOI] [PubMed] [Google Scholar]
- 4. Gattinori L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med. 2020;46:1099‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Japan ECMOnet for COVID‐19 . Nationwide system to centralize decisions around ECMO use for severe COVID‐19 pneumonia in Japan (special correspondence). J Intensive Care. 2020;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL‐6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med. 2006;260:429‐434. [DOI] [PubMed] [Google Scholar]
- 7. Hani C, Trieu NH, Saab I, et al. COVID‐19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101:263‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okada F, Ando Y, Honda K, Tanoue S, Matsumoto S, Mori H. Comparison of pulmonary CT findings and serum KL‐6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol. 2009;82:212‐218. [DOI] [PubMed] [Google Scholar]
- 9. Baque‐Juston M, Pellegrin A, Leroy S, Marquette CH, Padovani B. Organizing pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging. 2014;95:771‐777. [DOI] [PubMed] [Google Scholar]
- 10. Del Rio C, Malani PN. COVID‐19‐new insights on a rapidly changing epidemic. JAMA. 2020;323:1339‐1340. [DOI] [PubMed] [Google Scholar]


