Abstract
The coronavirus disease 2019 (COVID‐19) pandemic has spread to various regions worldwide. As of 27 April 2020, according to real‐time statistics released by the World Health Organization, there have been 84 341 confirmed cases and 4643 deaths in China, with more than 2 979 484 confirmed cases and 206 450 deaths outside China. The detection of antibodies produced during the immune response to severe acute respiratory syndrome coronavirus 2 infections has become an important laboratory method for the diagnosis of COVID‐19. However, at present, a little research on these specific antibodies has been conducted. In this study, a retrospective analysis was used to explore the dynamic changes of serum immunoglobulin M (IgM) and IgG antibody and factors affecting diagnostic efficacy, so as to provide a theoretical basis for clinical diagnosis and treatment.
Keywords: COVID‐19, IgG, IgM, SARS‐CoV‐2
Highlights
This is a retrospective analysis describing the dynamic changes of serum IgM and IgG antibody levels in patients with COVID‐19.
And the innovation point is analysis of factors (the severity of illness, whether the patient had symptomatic infection, albumin levels)influencing antibody levels on admission.
1. INTRODUCTION
In December 2019, patients with coronavirus disease 2019 (COVID‐19) began to appear in the city of Wuhan, Hubei province, China. 1 , 2 , 3 The epidemic rapidly spread to many other regions within China and then to regions across the globe. Although the situation in China is currently under control, the pandemic continues to spread in other countries, increasing the risk of overseas import of the disease to China.
COVID‐19 is caused by a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) 4 by the International Committee of Virus Classification. Although the detection of viral nucleic acid by reverse‐transcription polymerase chain reaction (RT‐PCR) is the gold standard for the diagnosis of SARS‐CoV‐2 infection, this method is complex and time‐consuming, with detection results easily affected by factors, such as collection time, sample type, and sample preservation, which increase the risk of false‐negative results. 5 Furthermore, this method is unable to meet the principles of early detection, early isolation, and early treatment, and is, therefore, not conducive to the prevention and control of the epidemic. As a product of the immune response to infection, antibody detection has the advantage of being easier and more convenient to conduct, more efficient, easier to preserve samples and having lower laboratory and personnel requirements. Diagnosis and treatment plan (trial 7th edition) clearly proposed that antibody detection is an important laboratory method for the diagnosis of COVID‐19. However, as SARS‐CoV‐2 is a novel virus, a thorough understanding of the dynamic changes of these antibodies in patients and influencing factors is required. SARS‐CoV‐2 belongs to the β‐coronavirus genus, sharing high‐sequence similarity (87.9% and 98.7%) with the SARS‐CoV. 6 , 7 Serum immunoglobulin M (IgM) antibodies against SARS‐CoV can be detected 3 to 6 days after infection, but levels rapidly decrease thereafter. Conversely, IgG antibodies appear, later on, approximately 8 days after the onset of symptoms, with levels increasing over the course of infection and remaining high for an extended period. 8 Therefore, it has been speculated that the dynamic change processes of SARS‐CoV‐2 antibodies may be similar to that of SARS‐CoV. To investigate this, the dynamic changes of IgM and IgG antibodies, factors affecting the positive seroconversion of IgM and IgG antibodies, and the quantitative levels of IgG antibodies were assessed in this study.
2. METHODS
2.1. Study participants
Approved by the Medical Ethics Committee of Taikang Tongji Hospital, Wuhan, samples were collected from a total of 802 inpatients diagnosed as COVID‐19 positive in Taikang Tongji Hospital from February to April 2020. According to the COVID‐19 diagnosis and treatment plan (trial 7th edition), positive diagnosis can be concluded if the nucleic acid test is positive, if the serum is positive for specific IgM and IgG antibodies, if serum IgG converts from negative to positive, or if antibody levels in the convalescent stage of infection are four or more times higher than that in the acute stage. All participants had a clear history of infection, either displaying typical clinical symptoms and/or were diagnosed by computed tomography angiography. Information on sex, age, clinical symptoms, comorbidity status, disease severity, course of the disease, IgM and IgG antibody levels, nucleic acid cycle threshold (C t) value, and albumin and total protein levels were collected. All experiments were performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki and it was approved by the Medical Ethics Committee of Taikang Tongji Hospital. Informed consent was obtained for any experiment on human subjects.
2.2. Antibody and nucleic acid detection
Fasting venous blood (2‐5 mL) was centrifuged, and the upper serum was separated and stored at −20°C. Serum IgM and IgG antibodies were quantitatively detected by the Axceed 260 (Bioscience, Tianjin, China) magnetic particle‐based chemiluminescence immunoanalyzer. Chemiluminescence signal‐to‐cutoff (S/co) values showed that S/co less than 1 was regarded as negative for the antibody and S/co greater than 1 as positive. Nasopharyngeal swab samples were collected and nucleic acid detection using model 7500 PCR gene amplification instrument (AMI). C t values of greater than 40 were considered negative for SARS‐CoV‐2 and values less than 40 were positive.
2.3. Statistical methods
Software SPSS26.0 was used for data analysis. Nonnormal variables were expressed as medians (Q1 and Q3) and were analyzed by the Mann‐Whitney U test. Counting data were compared using the χ 2 test. Correlation analysis was performed using the Spearman correlation coefficient. Data consistency was measured by calculating the Kappa coefficient using the Kappa consistency test. GraphPad Prism 8.0 was used to produce charts.
3. RESULTS
3.1. Basic patient information
Among the 802 patients, 58.48% were female with a median age of 63 years (interquartile range [IQR18], range: 13‐99 years). The median times for the following factors followed: virus shedding, 23 days (IQR22, range: 2‐61 days); hospitalization, 13 days (IQR10, range: 2‐55 days); and disease duration, 52 days (IQR21, range: 9‐101 days). 77.81% of patients had symptomatic infections, the main symptoms being fever (62.84%), cough (60.35%), and fatigue (43.39%). 53.74% of patients had at least one comorbidity, mainly hypertension (37.53%), diabetes (17.21%), or chronic heart disease (13.47%). On admission, 20.25% of the patients had decreased lymphocyte counts, and 64.47%, 17.78%, and 7.37% of patients had elevated D‐dimer, C‐reactive protein, and white blood cell counts, respectively. Patients with severe infections and deaths accounted for 29.05% and 1% of cases, respectively (Table 1).
Table 1.
Clinical and laboratory findings of patients on admission
| N (+) | Total | Positive (%) | |
|---|---|---|---|
| Clinical findings | |||
| Fever | 528 | 802 | 62.84 |
| Cough | 484 | 802 | 60.35 |
| Fatigue | 348 | 802 | 43.39 |
| Runny nose | 55 | 802 | 6.89 |
| Sore throat | 64 | 802 | 7.98 |
| Myalgia | 44 | 802 | 5.48 |
| Vomiting | 18 | 802 | 2.20 |
| Diarrhea | 58 | 802 | 7.23 |
| Dyspnea | 142 | 802 | 18.08 |
| Chest tightness | 226 | 802 | 28.17 |
| Shortness of breath | 145 | 802 | 18.08 |
| Comorbidity | 431 | 802 | 53.74 |
| Hypertension | 301 | 802 | 37.53 |
| Diabetes | 138 | 802 | 17.21 |
| Chronic heart disease | 108 | 802 | 13.47 |
| Chronic lung disease | 50 | 802 | 6.23 |
| Chronic cerebrovascular disease | 55 | 802 | 6.86 |
| Chronic kidney disease | 27 | 802 | 3.37 |
| Disease severity status | … | … | … |
| Light | 10 | 802 | 1.25 |
| General | 559 | 802 | 69.70 |
| Severe | 201 | 802 | 25.06 |
| Critical | 32 | 802 | 3.99 |
| Outcomes | … | … | … |
| Survival | 794 | 802 | 99.00 |
| Death | 8 | 802 | 1.00 |
| Laboratory findings | |||
| White blood cell count, ×109/L | … | … | … |
| <3.5 | 40 | 733 | 5.46 |
| 3.5‐9.5 | 639 | 733 | 87.18 |
| >9.5 | 54 | 733 | 7.37 |
| Lymphocyte count, ×109/L | … | … | … |
| <1.1 | 145 | 716 | 20.25 |
| 1.1‐3.2 | 553 | 716 | 77.23 |
| >3.2 | 18 | 716 | 2.51 |
| D‐dimer, ng/mL | … | … | … |
| <243 | 70 | 197 | 35.53 |
| >243 | 127 | 197 | 64.47 |
| C‐reactive protein, mg/L | … | … | … |
| <10 | 550 | 675 | 81.48 |
| >10 | 120 | 675 | 17.78 |
3.2. Dynamic analyses of SARS‐CoV‐2 antibodies and nucleic acid in patients
The positive rates of IgM, IgG, and IgM or/and IgG antibodies were 28.55% (229/802), 87.28% (700/802), and 88.40% (709/802), respectively, and the positive rate of nucleic acid was 70.32% (564/802). The positive rates of IgM and nucleic acid peaked within 1 week of disease onset, reaching 40% and 96.36%, respectively, and the positive rates of IgG, and IgM or/and IgG antibodies peaked after the ninth week of the disease, reaching 93.50% and 94.80%, respectively. Within 3 weeks of disease onset, the positive rate of nucleic acid was higher than that of IgM, IgG, and IgM or/and IgG; however, after the first 3 weeks, the positive rates of IgG and IgM or/and IgG antibodies were higher than that of nucleic acid, which showed a clear downward trend after 1 week (Figure 1A). The positive rate of IgM antibodies showed a downward trend 1 week after disease onset and continued to fluctuate after the fourth and eighth weeks. Conversely, the positive rates of IgG, and IgM or/and IgG antibodies showed an upward trend after the first week of disease onset (Figure 1B). Among the 564 nucleic acid‐positive samples, 516 were positive for IgM or/and IgG. Among the 238 samples that were negative for nucleic acid for the duration of the assessment, 193 were positive for IgM or/and IgG antibodies, although the Kappa value between these groups was only 0.126. Serum levels of IgM showed a downward trend after the first week and fluctuated in the fourth week, while IgG antibody levels showed an upward trend from the first week to the fifth week (Figure 2).
Figure 1.
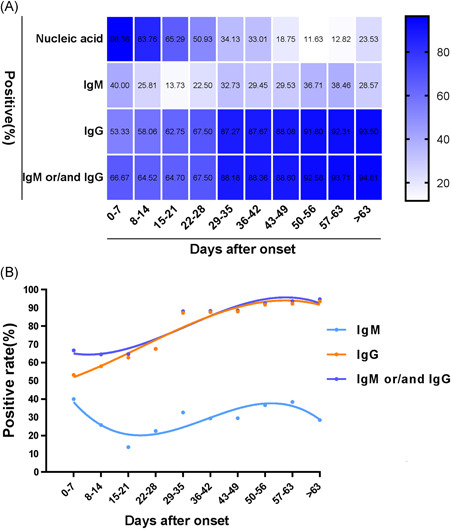
Dynamic changes in the positive rates in patients after the onset of disease. A, Heat map of changes in the positive rates of antibodies. B, Changes in the positive rates of nucleic acid and antibodies at different time periods after coronavirus disease 2019 onset. IgM, immunoglobulin M
Figure 2.
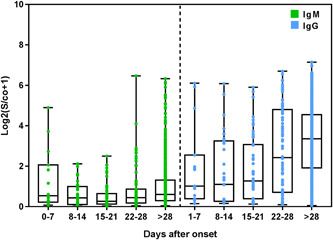
Dynamic changes of antibody levels in patients after the disease onset. IgM, immunoglobulin M
3.3. Analysis of factors influencing antibody levels on admission
3.3.1. The relationship between the positive rates of IgM and IgG antibodies on admission and patient sex
The patients were divided into female (n = 469) and male (n = 333) groups. On admission, the positive rates of IgM antibodies in the female and male groups were 53.71% and 46.29%, respectively, and the positive rates of IgG antibodies in these groups were 58.86% and 41.14%, respectively. Although the positive rates of IgM and IgG detection in the female group were higher than those in the male group on admission, these differences were not statistically significant (P > .05).
3.3.2. The relationship between the positive rates of IgM and IgG antibodies on admission and patient comorbidity status
The patients were divided into noncomorbidity (n = 371) and comorbidity (n = 431) groups. The positive rate of IgM antibodies in the comorbidity group (52.84%) was higher than that in the noncomorbidity group (47.16%). The positive rate of IgG antibodies in the comorbidity group (53.29%) was also higher than that in the noncomorbidity group (46.71%), but the difference was not statistically significant (P > .05).
3.3.3. The relationship between the positive rates of IgM and IgG antibodies on admission and whether the patient had a symptomatic infection
The patients were divided into asymptomatic (n = 178) and symptomatic (n = 624) infection groups. The positive rates of IgM and IgG antibodies in the symptomatic infection group (95.63% and 95.57%, respectively) were significantly higher than those in the asymptomatic infection group (4.37% and 4.43%, respectively), but only the difference between IgG detection was statistically significant (P < .05).
3.3.4. The relationship between the positive rate of IgM and IgG antibodies on admission and the severity of illness
To classify the severity of the illness of participants, those displaying mild and normal symptoms were classified as the “non‐severe group,” and severe and critically ill patients were classified as the “severe group.” The positive rates of IgM and IgG antibodies in the nonsevere group (71.62% and 71.14%, respectively) were higher than those in the severe group (28.38% and 28.86%, respectively), but these differences were not statistically significant (P > .05).
3.3.5. The relationship between IgG antibody levels on admission and sex, comorbidity status, symptoms, and severity of illness
IgG antibody levels in compared groups varied as follows: significantly higher in males than females, 10.75 S/co (IQR23.79) and 6.82 S/co (IQR17.48), respectively; significantly higher in patients with comorbidity than patients without, 9.84 S/co (IQR23.53) and 6.58 S/co (IQR17.89), respectively; significantly higher in symptomatic patients than asymptomatic patients, 8.48 S/co (IQR20.70) and 2.96 S/co (IQR12.48), respectively; and significantly higher in the severe patient group than nonsevere patient group, 11.05 S/co (IQR24.65) and 7.04 S/co (IQR18.50), respectively. All differences were statistically significant (P < .05; Figure 3).
Figure 3.
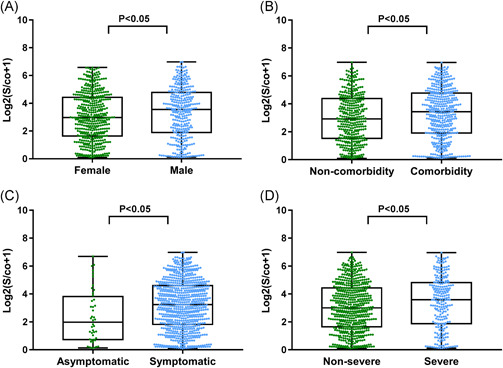
The influence of sex, comorbidity status, symptoms, and the severity of illness on immunoglobulin G (IgG) antibody levels. Comparison of IgG antibody levels and (A) sex, (B) comorbidity and noncomorbidity groups, (C) symptomatic and asymptomatic groups, and (D) nonsevere and severe groups
3.3.6. The relationship between IgG antibody levels and age, albumin levels, and total protein levels on admission
IgG antibody levels correlated positively with age (P < .01), negatively with albumin levels (P < .05), and showed no correlation with total protein levels (P > .05; Figure 4).
Figure 4.
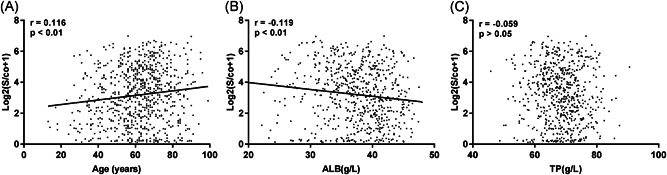
Correlation analysis of immunoglobulin G (IgG) antibody levels with (A) age, (B) albumin (ALB) levels, and (C) total protein (TP) levels
4. DISCUSSION
The COVID‐19 epidemic has rapidly developed into a serious global situation, threatening the physical and mental health of millions across the globe. 9 , 10 The median age of the 802 patients included in this study was 63 years, with slightly more females than men. 53.74% of the patients had at least one comorbidity, such as hypertension, diabetes, or chronic heart disease, which indicates that the elderly and patients with comorbidity were more susceptible to COVID‐19.
While 65.85% of patients had a fever, 34.16% of the patients had no fever during the course of the disease, highlighting the importance of testing suspected cases even in the absence of a fever to prevent missed diagnosis. Other than fevers, the most common symptoms were cough and fatigue, which are similar to some SARS‐CoV infection symptoms 11 . However, other SARS‐CoV infection symptoms, such as sore throat, runny nose (upper respiratory tract infection symptoms), vomiting, and diarrhea were rarely seen, which indicates that SARS‐CoV‐2 target cells mainly exist in the lower respiratory tract.
Upon admission to the hospital, 20.25% of the patients displayed reduced lymphocyte counts, which indicates that SARS‐CoV‐2 may attack lymphocytes to reduce the immunity of infected individuals, allowing SARS‐CoV‐2 to replicate in large quantities in the body. This study also found that 17.78%, 64.47%, and 7.37% of patients had elevated C‐reactive protein, D‐dimer, and white blood cells, respectively. It is well known that C‐reactive protein is an indicator of inflammatory response and increases during bacterial or viral infections. Also, D‐dimer is an indicator of blood agglutination. Studies have shown that blood hypercoagulability is associated with patients with severe infections, particularly those with respiratory distress syndrome. The level of D‐dimer will continue to increase in severe patients with respiratory distress syndrome. 9
Lan et al 12 found that some patients who met the discharge criteria were repositive for nucleic acid a few days after discharge. The results of this study are similar to those of the study conducted by Lan et al, with some discharged patients who were readmitted to hospital testing positive for nucleic acid. The median time for virus shedding was 23 days, with 2 and 61 days being the shortest and longest periods, respectively. Statistical analysis of virus shedding times provides a reference for the length of isolation needed by discharged patients to prevent them from contacting family and friends. As some patients did not have detectable nucleic acid during the length of observation, this may have affected the overall virus shedding times we measured in this study.
IgM is the first antibody produced, lasting for a short period in the body before rapidly decaying. The results of this study show that the positive rate of IgM antibodies peaks within a week, decreases during the following week, before rising again and fluctuating in the fourth and eighth weeks. IgM antibody levels also decrease after 1 week and fluctuate in the fourth week. These findings demonstrate that IgM antibodies can be used to indicate current or recent infections, or recurrent infections, which are thought to arise when a patient fails to clear the virus completely during the convalescent period.
Nucleic acid detection is complex and time‐consuming, with detection results easily affected by factors such as collection time, sample type, sample treatment, and specimen preservation, which increase the risk of false‐negatives. Conversely, antibody detection has the advantage of being easier and more convenient to conduct, more efficient, easier to preserve samples, and having lower laboratory and personnel requirements, thereby reducing the risk of false negatives. In this study, the positive rate of nucleic acid was only 70.32%, while the positive rate of antibodies was 88.40%. The positive rate of nucleic acid decreased after the first week, while the positive rates of IgM or/and IgG increased after 1 week. Furthermore, the positive rate of nucleic acid was high in the first 3 weeks, while the positive rate of antibody was higher than that of nucleic acid after 3 weeks. Among the 238 nucleic acid negative samples, 193 were positive for IgM or/and IgG, with a positive rate of 81.09%. The consistency test between nucleic acid and IgM or/and IgG antibody results showed that the Kappa value of both was only 0.126, which may be because the nucleic acid‐positive results were mainly concentrated in the early and middle stages of the disease, while the positive antibody results were mainly concentrated in the middle and latter stages. This demonstrates that antibody detection can be used as an auxiliary diagnostic method for nucleic acid testing, and nucleic acid detection can supplement the low sensitivity of antibody detection in the early stages of infection. Combined nucleic acid and antibody detection could potentially improve diagnostic efficiency, reducing the risk of missed diagnosis.
The positive rate of IgG antibodies in patients with asymptomatic infections was significantly lower than that in patients with symptomatic infections, the positive rate of which was less than 5%. It is speculated that asymptomatic infections may affect IgG antibody conversion. Therefore, asymptomatic patients with a clear history of the disease should not be deemed clear of infection even if the antibody and nucleic acid screenings are negative; the continued monitoring of nucleic acid and antibody levels for several days is necessary to avoid missed diagnosis. On admission, the positive rate of IgG antibodies in nonsevere patients was significantly higher than that in severe patients (P < .05). This implies that the severity of the disease affects the conversion of IgG antibodies. If the IgG antibody level is high, the level of albumin will decrease accordingly. As albumin is often used as an indicator of the body's nutritional status, this decrease suggests that the increased antibody production during infection may consume nutrients. IgG is a protective antibody and it may, therefore, be necessary to closely monitor albumin levels of patients with COVID‐19. If the albumin level decreases, intervention may be necessary to ensure the body can produce enough antibodies to battle and recover from the disease. The results of this study also showed that sex, comorbidity status, symptoms, the severity of illness, and age all affected the levels of IgG antibodies; these factors will all likely influence the accuracy of diagnosis.
In this study, we described the dynamic changes of antibody seroconversion and discussed factors that could affect the diagnostic efficacy of antibodies. Antibody detection can be used as a supplementary diagnostic tool for nucleic acid testing. The improved diagnostic efficiency of COVID‐19 by this combined approach could have important clinical applications.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
MY and NW contributed to data analysis, technical graphics, and writing of this paper. MY, NW, YZ, YS, LW, and LL contributed to data collection and graphics processing. JL and XT contributed to the editing of this paper.
ACKNOWLEDGMENTS
This study was supported by research grants from the Natural Science Foundation of Liaoning Provincial (Grant No. 2019‐ZD‐1027) and Novel Coronavirus Special Project of Taikang Tongji Hospital, Wuhan (Grant No. TKTJKY2020069).
Yan M, Zheng Y, Sun Y, et al. Analysis of the diagnostic value of serum specific antibody testing for coronavirus disease 2019. J Med Virol. 2021;93:441–447. 10.1002/jmv.26230
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome‐related coronavirus: the species and its viruses—a statement of the coronavirus study group. bioRxiv. 2020. [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortega VE, Li X, O'neal WK, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;201:540‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22(2):74‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee HK, Lee BH, Seok SH, et al. Production of specific antibodies against SARS‐coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J Vet Sci. 2010;11(2):165‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709‐710. [DOI] [PubMed] [Google Scholar]
- 11. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 12. Lan L, Xu D, Ye G, et al. Positive RT‐PCR test results in patients recovered from COVID‐19. JAMA. 2020;323:1502‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]


