After the World Health Organisation declared Coronavirus Disease 2019 (COVID‐19) a global pandemic on March 11, 2020, it became clear that health professionals worldwide were facing a severe and unprecedented challenge. On March 28, the FDA issued an emergency use authorisation order for use of chloroquine and hydroxychloroquine in COVID‐19 patients, and on May 1, 2020, the agency gave the same authorisation for use of remdesivir. Even though different treatment strategies are undergoing evaluation, particularly for severe COVID‐19 cases requiring intensive care, the consensus regarding best treatment approach has not yet been reached.
Earlier this year, Zhe Xu and colleagues provided a detailed case report of a 50‐year‐old man with COVID‐19 in the Lancet Respiratory Medicine journal. 1 This case is particularly interesting since it provides a detailed day‐to‐day account of the hospitalisation, laboratory parameters and histological findings in a patient who neither belonged to a very high‐risk age group, nor did he have any other co‐morbidity. 2
The patient's assessment revealed an overactivation of T cells manifested by an increase in the Th17 subset of CD4+ T cells leading to increased production of IL‐17 and IL‐22 cytokines which in turn caused the cytokine release storm (CRS) with a rapid and severe deterioration of the patient's condition. 1 Pathologic postmortem lung analysis revealed a high number of Th17 lymphocytes in alveolar spaces. Besides the referenced case report, there is emerging body of evidence supporting the role of IL‐17 in the pathogenesis of severe COVID‐19 disease, including two recent publications reviewing the immune response in patients with COVID‐19, that further emphasise the Th17‐type cytokine storm in pathogenesis of the disease. 3 , 4 Wu and Yang highlighted the Th17 response in CRS of COVID‐19 and proposed using fedratinib, a Janus kinase 2 (JAK2) inhibitor which blocks downstream cellular Th17 signalling pathway, in the treatment of CRS of COVID‐19 patients. 3 However, in our view, JAK inhibition of intracellular enzymes would also be associated with significant disadvantages entailing unintended and/or off‐target effects leading to difficulty in predicting JAK2 inhibitors' biological effects. 5
Since the increase in Th17 CD4+ T cells is also found in several inflammatory diseases, such as in severe plaque psoriasis, 6 we hypothesise that the use of IL‐17 blocking agents could be beneficial in the treatment of severe COVID‐19 cases.
The ligation of IL‐17 to IL‐17RA initiates activation of transcription factors NFκB, IκBζ, API and C‐EBP, which potentiate IL‐17–induced transcription of the most potent proinflammatory cytokines (TNF‐α, IL‐1β, IL‐6, IL‐8, G‐CSF and GM‐CSF), chemokines involved in attracting and further recruitment of immune infiltrates (IL‐8, CXCL1, CXCL2, CXCL5, CCL2, CCL7 and CCL20), and matrix metalloproteinases involved in tissue damage (MMP1, MMP3, MMP9, MMP12 and MMP13). 5 , 7 IL‐17 has been demonstrated in blood and affected tissues of patients with COVID‐19, and IL‐6 levels were significantly increased on Days 2 and 3 of the patient's hospitalisation 1 ; thus, IL‐17 inhibitors, which block IL‐6 and IL‐8 secretion from innate immune system, like neutrophils, mast cells, γδ T cells and innate lymphoid cells (ILC3), could prove as safe and effective therapy for severe COVID‐19 (Figure 1). A recent study by Liu et al. also revealed an increased IL‐6 level as well as increased levels of several other proinflammatory Th1, Th2 and Th17 cytokines (e.g., IL‐2, 4, 6, 7, 10, 12 and 17) in patients with severe COVID‐19. 8 Blockade of IL‐17 alone has been shown as clinically effective in many circumstances and diseases, despite the presence of myriad of proinflammatory cytokines; nevertheless, relevance of mediators crucial for the CRS response remains to be elucidated. 9 , 10
FIGURE 1.
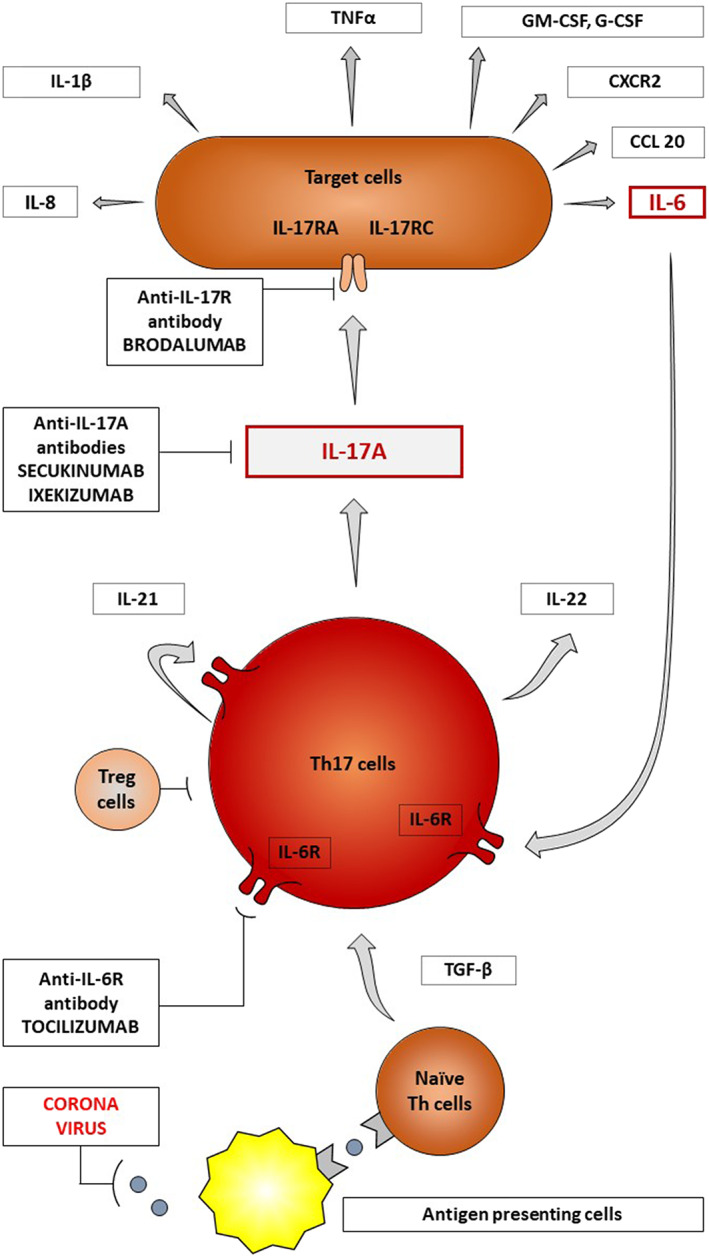
T helper 17 (Th17) cells are differentiated from naïve precursor T cells in response to coronavirus presentation, after co‐stimulation by antigen presenting cells and transforming growth factor‐β (TGF‐β) secretion. Th17 cells produce interleukin‐17A (IL‐17A) causing wide spectrum cell activation (e.g., dendritic cells, endothelial cells and fibroblasts). IL‐17 contributes to cytokine release storm by stimulating excessive and uncontrolled production of proinflammatory interleukins (e.g., IL‐1β, IL‐6 and IL‐8), tumour necrosis factor‐alpha, colony stimulating factors and chemokines. Secukinumab and ixekizumab are immunomodulators that block IL‐17A and could be beneficial in therapy of severe COVID‐19. Brodalumab is directed against interleukin‐17 receptor A (IL‐17RA), another potential target molecule in therapy of severe COVID‐19. CCL20, chemokine CC motif ligand 20; CXCR2, CXC chemokine receptor 2; G‐CSF, granulocyte colonystimulating factor; GM‐CSF, granulocyte‐macrophage CSF; IL, interleukin; R, receptor; Th17 cells, T helper 17 cells; Treg, regulatory T cells; TGF‐β, transforming growth factor‐ β;TNF‐α, tumour necrosis factor‐alpha
IL‐17 specifically boosts proinflammatory, but not antiviral gene expression in human cells infected with respiratory viruses by stimulating non immune cells (fibroblasts and epithelial cells) to produce increased amounts of proinflammatory cytokines and chemokines in response to viral infections that attract other immune cell types (for example, neutrophils) which can lead to increased morbidity while simultaneously remaining inefficient in preventing the pathogen's spread. 11
To date, there have been three IL‐17 blocking agents available: secukinumab, ixekizumab and brodalumab. 5 Secukinumab and ixekizumab are monoclonal IgG1 antibodies that bind specifically to IL‐17A and that have been demonstrated to be very effective in the treatment of severe plaque psoriasis. 12 Both drugs have a quick onset of action, high efficacy, tolerability and a well‐established safety profile which does not include a decrease in the lymphocyte count. 5 The substantial decrease in the total number of lymphocytes was found in patients with severe COVID‐19, and this seems to facilitate viral replication. 13 In contrast to TNF‐α inhibitors, IL‐17 blocking agents are not associated with a decrease in lymphocyte count. 14
Brodalumab, a human monoclonal IgG2κ antibody against IL‐17 receptor A (IL‐17RA), was shown to be superior to placebo and ustekinumab for psoriasis treatment, due to complete blockade of key mediators of the T helper 17 pathway. 5 Brodalumab as an IL‐17 receptor blocker could potentially offer improved efficacy; however, patient suicides reported during the AMAGINE trial involving brodalumab raised some concerns regarding the safety of this agent, even though causal relationship was not unequivocally demonstrated. 5
We believe that secukinumab, a fully human monoclonal antibody which exhibits significantly lower immunogenicity potential in patients with moderate‐to‐severe plaque psoriasis compared to ixekizumab (a humanised monoclonal antibody), would be best suitable for the treatment of severe COVID‐19 patients. 15 , 16
IL‐17 blocking agents can suppress the production of several Th17 signature cytokines (including the effects of IL‐6 and TNF‐α on other types of cells), thus theoretically offering significant therapeutic potential for Th17‐associated CRS in COVID‐19. Owing to their parenteral administration, these drugs could also be used concomitantly with other antiviral drugs as well as in patients on ventilator support.
Preclinical studies demonstrated that the response to severe swine‐origin influenza A virus H1N1 (S‐OIV) in mice was boosted by introduction of IL‐17 blocking agents, which led to reduction in cell and fluid infiltrates and an improvement in severity of acute lung injury. 8
Crowe et al. found IL‐17RA signalling crucial for acute lung injury in response to pulmonary influenza infection. This finding suggests that therapeutic modulation of IL‐17 signalling might be beneficial in treating immunopathology associated with pulmonary viral infections. 17
Li et al. demonstrated that pandemic H1N1 influenza virus caused acute lung injury in an IL‐17‐dependent manner in mice. 18 They also reported that the response to pandemic H1N1 influenza virus in mice was improved by IL‐17‐blocking agents, which reduced disease duration, as well as cellular and fluid infiltrates. IL‐17 blocking agents decreased pulmonary recruitment of inflammatory cells, cytokine production by T cells and the formation of lung oedema without preventing virus clearance in mice. 18 These broad beneficial effects support further the idea that IL‐17 blocking agents might be tested as potential treatment in severe COVID‐19 patients.
Tocilizumab, a recombinant human monoclonal IgG1 antibody that specifically binds to soluble and membrane‐bound IL‐6 receptors, has been shown to be effective in the treatment of severe COVID‐19 by blocking IL‐6 signalling and its proinflammatory response. 19 , 20 Until now, several clinical trials have been registered in order to assess safety and efficacy of tocilizumab in the treatment of severe COVID‐19 pneumonia in adult patients. 19 , 20 Since IL‐17 inhibitors and tocilizumab share the common Th17 signalling pathway, IL‐17 blocking agents could also prove as safe and effective therapy for severe COVID‐19. They might be even more effective than tocilizumab, since IL‐17 precedes synthesis of potent proinflammatory cytokines IL‐6 and TNF‐α. Moreover, subcutaneous route of administration of IL‐17 blocking agents could prove more convenient than intravenous administration of tocilizumab.
In the treatment of severe plaque psoriasis, secukinumab is administered subcutaneously in the dose of 300 mg once weekly during the first month of treatment, followed by a dose of 300 mg every 4 weeks. Ixekizumab is also administered subcutaneously 160 mg every 2 weeks during the first 3 months, followed by a dose of 160 mg every 4 weeks. The recommended brodalumab dose in the treatment of severe plaque psoriasis is 210 mg once weekly during the first 2 weeks of treatment, followed by a dose of 210 mg every 2 weeks administered subcutaneously.
It is worth noting that IL‐17 blocking agents have not yet been evaluated in clinical trials for severe COVID‐19 and patients would not receive the same dosage as patients with severe plaque psoriasis. Also, the timing of IL‐17 blocking agent administration to severe COVID‐19 patients would be crucial. It would perhaps be best to begin the treatment in the middle stage of CRS, when a significant increase in IL‐6 levels is to be expected. 1 , 21
While we currently do not have a vaccine or highly effective antiviral treatments for COVID‐19, repurposing certain immunomodulators, such as IL‐17 blocking agents, might help to prevent CRS in severe COVID‐19 cases, thereby further improving patients' outcomes. Finally, in our new, COVID‐19 directed worldwide reality and with the constraints of limited experimental evidence regarding IL‐17 blocking agents in mind, the importance of drug repurposing and effective dosing scheme investigation through conduct of well designed, randomised and double blind controlled clinical trials cannot be overstated enough.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All authors have made equal contributions to the article.
REFERENCES
- 1. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome [published online ahead of print 2020 Feb 18]. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruan S. Likelihood of survival of coronavirus disease 2019 [published online ahead of print 2020 Mar 30]. Lancet Infect Dis. 2020;20(6):630‐631. 10.1016/S1473-3099(20)30257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib [published online ahead of print, 2020 mar 11]. J Microbiol Immunol Infect. 2020. S1684–1182(20)30065–7;53(3):368‐370. 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schett G, Sticherling M, Neurath MF. COVID‐19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271‐272. 10.1038/s41577-020-0312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645‐653. 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2(1):16082–16099. 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- 7. de Ruiz de Morales JMG, Puig L, Daudén E, et al. Critical role of interleukin (IL)‐17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19(1):102429–102444. 10.1016/j.autrev.2019.102429 [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, Zhang Z, Qin Y, Li X, Zhao D, Li S, Tan S, Wang Z, Li J, Shen C, Li J, Peng L, Wu W, Cao M, Xing L, Xu Z, Chen L, Zhou C, Liu WJ, Liu L, Jiang C Elevated plasma level of selective cytokines in COVID‐19 patients reflect viral load and lung injury [published online ahead of print, 2020 Mar 9]. Natl Sci Rev 2020;nwaa037. 10.1093/nsr/nwaa037, 7, 6, 1003, 1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldmann M, Maini RN, Woody JN, et al. Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. Lancet. 2020;395(10234):1407‐1409. 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. IL‐17 boost proinflammatory outcome of antiviral response in human cells. J Immunol. 2011;187(10):5357‐5362. 10.4049/jimmunol.1100917 [DOI] [PubMed] [Google Scholar]
- 12. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. 10.1038/S41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study [published online ahead of print 2020 Jan 29]. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu WJ, Zhao M, Liu K, et al. Tcell immunity of SARSCoV: implications for vaccine development against MERSCoV. Antiviral Res. 2017;137:82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti‐interleukin‐17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2017;176(3):752‐758. 10.1111/bjd.14965 [DOI] [PubMed] [Google Scholar]
- 16. Spindeldreher S, Maillère B, Correia E, et al. Secukinumab demonstrates significantly lower immunogenicity potential compared to Ixekizumab [published correction appears in Dermatol Ther (Heidelb). 2018 mar 17]. Dermatol Ther (Heidelb). 2018;8(1):57‐68. 10.1007/s13555-018-0220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crowe CR, Chen K, Pociask DA, et al. Critical role of IL‐17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301‐5310. 10.4049/jimmunol.0900995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Yang P, Sun Y, et al. IL‐17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22(3):528‐538. 10.1038/cr.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393–108398. 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814–818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, Wu Z, Li J‐W, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954–105960. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]


