Whilst the majority of patients with COVID‐19 infection have mild self‐limiting symptoms, for some the SARS‐CoV2 virus can trigger a severe hyperinflammatory syndrome that is life‐threatening. Anti‐IL6 therapy has shown promise in restraining this hyperinflammatory syndrome and whilst IL‐6 is a pleiotropic mediator of the inflammatory response, redundancy within inflammatory pathways means that the use of such targeted monoclonal therapy may have too restricted a repertoire in some patients. We present the case of a 53‐year‐old haematopoetic stem cell transplant recipient who developed severe COVID‐19 that was refractory to anti‐IL6 therapy, but responded to JAK/STAT inhibition with ruxolitinib, demonstrating its safety and efficacy in this setting.
For the majority of patients, the natural course of COVID‐19 infection is mild and self‐limiting, but for some, the disease is severe and can be catastrophic. 1 There is mounting evidence that a viral‐induced cytokine storm can drive a hyperinflammatory syndrome, and raised serum ferritin and IL‐6 levels are associated with a worse outcome. 2 Whilst this phenomenon is not unique to the SARS‐CoV‐2 virus, a striking feature in this setting is the hypoxia and lung injury associated with it. Patients who develop respiratory failure and require ventilatory support have higher plasma levels of an array of inflammatory cytokines including IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα, and their presence is associated with an inferior outcome. 3 The manifestation of the COVID‐19 hyperinflammatory syndrome is reminiscent of the cytokine release syndrome seen with chimaeric antigen receptor (CAR) T‐cell therapy, and this has provoked the use of anti‐IL6 therapy with promising results. 4 , 5 However, this approach directs therapy again a single facet of the immune response, and whilst IL‐6 is a pleiotropic mediator of inflammatory signalling, this strategy may be too targeted. There is considerable redundancy in cytokine and inflammatory pathways, so inhibiting a single molecule may permit collateral pathways to continue to drive the hyperinflammatory response.
Ruxolitinib is a JAK1/JAK2 inhibitor indicated for the treatment of disease‐related splenomegaly or constitutional symptoms in patients with myelofibrosis or polycythaemia vera. Whilst ruxolitinib also significantly reduces serum IL‐6 levels and C‐reactive protein (CRP) in patients with myelofibrosis, 6 the central role of the JAK/STAT pathways as downstream effectors of the inflammatory response means the end‐effector responses are more far‐reaching. JAK1 and JAK2 are critical regulators of IL‐2, IL‐5, IL‐10 as well as many other cytokines implicated in the inflammatory response seen in COVID‐19. 3 , 7 This, coupled with evidence of a rapid mode of action, with reports of cytokine‐induced STAT3 phosphorylation being inhibited within 2 hours of administration, 8 means ruxolitinib may offer therapeutic potential in COVID‐19 hyperinflammation.
We report the case of 53‐year‐old man who had a haploidential stem cell transplant for chronic myeloid leukaemia in blast phase in 2017. His post‐transplant course had been complicated by both acute and chronic graft‐versus‐host disease requiring multiple treatments including corticosteroids, infliximab, etanercerpt and mesenchymal stromal cells, but was most recently quiescent with corticosteroid, ciclosporin and mycophenolate maintenance. He had a history of diabetes which had been poorly controlled recently because of ongoing corticosteroid therapy, but otherwise had a good performance status. He presented with a five‐day history of dry cough and breathlessness, and on admission was breathless at rest, with a respiratory rate of 20 and oxygen saturation of 95% on air. His chest X‐ray showed basal and patchy peripheral ground‐glass shadowing, consistent with mild COVID‐19, and a throat swab was positive for SARS‐CoV‐2 by polymerase chain reaction (PCR). Despite empirical antibiotics, antifungals and intermediate dose low molecular weight heparin (LMWH) as per institutional protocol, over the next 48 hours he deteriorated significantly with tachypnoea, increasing oxygen requirements and progressive ground‐glass shadowing on X‐ray. He had evidence of an inflammatory response with raised D‐dimers of 1146 μg/l and ferritin of 4191 μg/l, although his CRP was only mildly elevated at 33 mg/l. He received two doses of 8 mg/kg tocilizumab (Roche Products Ltd, Welwyn Garden City, UK) 24 hours apart and whilst there was a rapid and sustained fall in his CRP, his ferritin continued to rise to 4600 μg/l, and his respiratory function continued to deteriorate. Repeat imaging showed progressive ground‐glass changes consistent with severe COVID‐19, and he required high flow oxygen (OptiflowTM) with 60% inspired oxygen concentration (FiO2). Given the progressive disease he received 5 mg twice daily of ruxolitinib (Novartis Pharmaceuticals UK Ltd, London, UK), which was escalated to 10 mg twice daily after six doses, by which time he required continuous positive airway pressure (CPAP). Following the introduction of 10 mg ruxolitinib, there was a contemporaneous improvement in respiratory function, allowing for a reduction in the level of respiratory support from CPAP to Optiflow, then to face mask and subsequently nasal cannula, with a corresponding reduction in FiO2 (Fig 1A), and a gradual decline in his serum ferritin (Fig 1B). No concurrent association could be made with any antibiotic, antifungal or other factor. His platelets were 77 × 109/l on the day of initiating ruxolitinib and did not fall below 50 × 109/l for the duration of treatment. He did not develop cytopenias in any other haematopoetic lineages and liver and renal function remained stable. Despite concomitant intermediate dose LMWH, he did not manifest any haemorrhagic complications. He continued to recover and was discharged home 28 days after admission, having completed 21 days of ruxolitinib, with no flare of symptoms on cessation.
Fig 1.
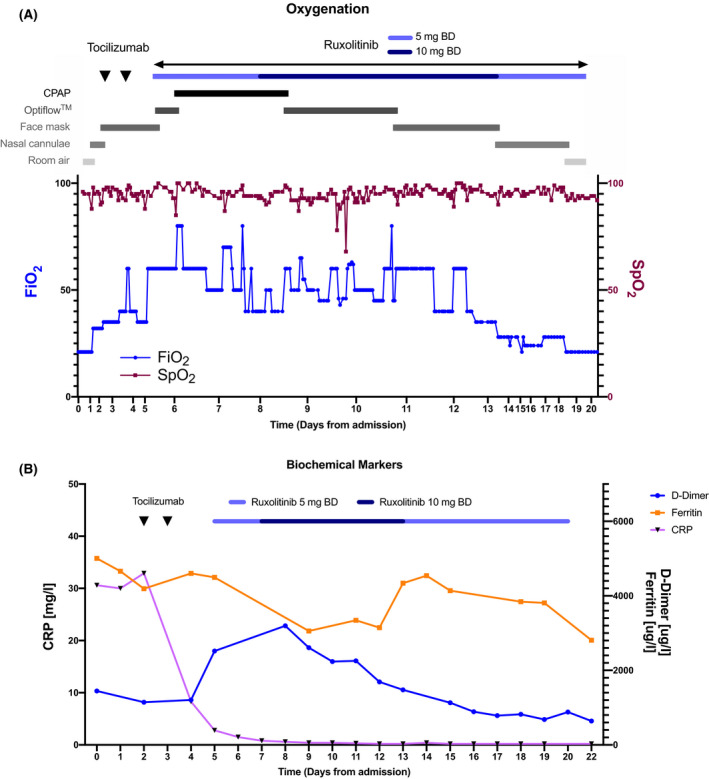
(A) Clinical observations and oxygen requirement during hospital admission and treatment with tocilizumab and ruxolitinib. (B) Biochemical parameters over the same duration. BD, bis in die (twice a day); CPAP, continuous positive airway pressure; CRP, C‐reactive protein; FiO2, inspired oxygen concentration, SpO2, oxygen saturation.
Our report suggests that patients with evidence of a hyperinflammatory response in severe COVID‐19 resulting in respiratory failure that does not respond to IL‐6 blockade may respond to biological agents targeting alternative components of the inflammatory cascade. Interestingly, despite clear evidence of an inflammatory response to SARS‐CoV‐2 this patient had a disproportionately raised ferritin:CRP ratio, which may be accounted for by his existing immunosuppression, and may also identify a group of patients in whom more promiscuous immunosuppression is necessary rather than targeted IL‐6 blockade.
Conflicts of interest
EB is an employee of F. Hoffmann‐La Roche Ltd. The other authors have no conflicts of interest to disclose.
Author contributions
AJI, LBC, SM, EB, CCT, GS, JFA and DM were involved in caring for the patient and contributed to data collection and analysis. AJI and DM wrote the manuscript, and all authors reviewed, edited and approved the final draft.
Acknowledgements
We thank the nurses, doctors and allied healthcare professionals in the haematology and CPAP units at Hammersmith Hospital. AJI and JFA acknowledge support from the NIHR and Imperial Biomedical Research Centre (BRC).
References
- 1. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaidos A, Katsarou A, Mustafa C, Milojkovic D, Karadimitris A. Interleukin 6‐blockade treatment for severe COVID‐19 in two patients with multiple myeloma. Br J Haematol. 2020;2019:2019–21. [DOI] [PubMed] [Google Scholar]
- 6. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double‐blind, placebo‐controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schindler C, Levy DE, Decker T. JAK‐STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. [DOI] [PubMed] [Google Scholar]
- 8. Eghtedar A, Verstovsek S, Cortes JE, Estrov Z, Burger, JA & Bivins, C , et al. Phase II study of the JAK2 inhibitor, ruxolitinib, in patients with refractory leukemias including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2010;116:4614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


