To the Editor,
There is scarce knowledge of “severe acute respiratory syndrome coronavirus 2” (SARS‐CoV‐2) infection in children. Its incidence appears to be lower than in adults (1%‐5% of the total cases). Very few publications include severe children requiring intensive care. 1 , 2 In this report, we describe the clinical course of a previously healthy infant admitted to the Pediatric Intensive Care Unit (PICU) with severe pneumonia. SARS‐CoV‐2 and Streptococcus pneumoniae were isolated from pleural effusion.
An 8‐months‐old healthy and correctly immunized male presented to the emergency department (ED). He had five‐days of fever, cough, nasal congestion, and increased work of breathing over the previous hours. Chest auscultation showed hypoventilation with bilateral crackles. Oxygen therapy through nasal cannula was started, blood test including blood culture was performed and intravenous cefotaxima (200 mg/kg/d) was administered. Chest X‐ray (CXR) revealed a left lower lobe (LLL) opacity and small pleural effusion (Figure 1). Laboratory findings showed increased levels of C‐reactive protein (CRP), procalcitonin (PCT), ferritin, and interleukin‐6 (IL‐6; Table 1). Reverse transcription‐polymerase chain reaction (RT‐PCR) on nasopharyngeal swab (NPS) for SARS‐CoV‐2 revealed positive. His clinical condition deteriorated, he was transferred to the PICU. On admission, high flow nasal cannula (HFNC) oxygen therapy was initiated. There was no improvement so noninvasive ventilation (NIV) was started. Empirical drugs for coronavirus disease‐2019 (COVID‐19) were initiated (azithromycin 10 mg/kg/d, lopinavir/ritonavir 12/3 mg/kg/12 h, and hydroxychloroquine 6.5 mg/kg/12 h).
Figure 1.
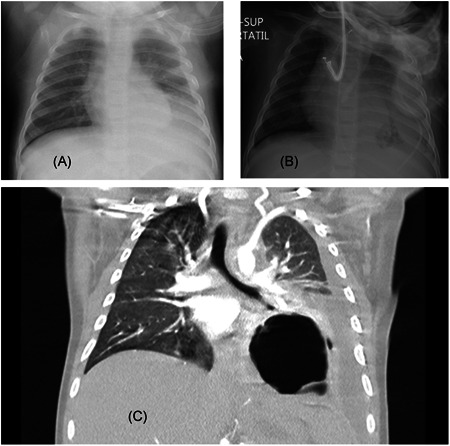
Radiologic findings. A, Chest X‐ray shows left lower lobe (LLL) opacity and small pleural effusion; B, Chest X‐ray after insertion of chest tube showed left sided pneumonia and remaining pleural effusion; C, chest computed‐tomography described LLL consolidation with a rounded well‐limited cavity compatible with necrotizing pneumonia and associated pleural effusion
Table 1.
Clinical course, laboratory findings, and treatment
| Days from PICU admission | |||||
|---|---|---|---|---|---|
| Day 1 (PICU admission) | Day 3 (pleural drainage) | Day 7 (discharge to ward) | Day 9 (PICU readmission) | Day 18 (discharge home) | |
| WBC/μL | 9640 | 13 910 | 12 030 | 42 880 | 18 070 |
| Neutrophils/μL | 6230 | 9000 | 4990 | 34 160 | 5790 |
| Lymphocytes/μL | 6239 | 3880 | 6180 | 6130 | 10 740 |
| Platelets/μL | 289 000 | 127 000 | 866 000 | 145 7000 | 777 000 |
| CRP (range, 0.01‐1 mg/dL) | 40 | 25.9 | 1.47 | 18.72 | 1.5 |
| PCT (range, 0.1‐0.5 ng/mL) | 5.3 | 3.85 | 0.22 | 0.14 | 0.16 |
| IL‐6 (range, 0‐7 pg/mL) | 303 | ND | ND | 139 | ND |
| Ferritin (range, 7‐140 ng/mL) | 484 | 395 | ND | ND | 159 |
| RT‐PCR SARS‐CoV‐2 | Positive | Positive (pleural fluid) | ND | Positive | Negative |
| Antibiotic therapy | Cefotaxime | Cefotaxime Clindamycin | Cefotaxime Clindamycin | Meropenem Linezolid | Linezolid |
| Hydroxychloroquine (day of treatment) | 1 | 3 | NR | Reintroduced | NR |
| Azithromycin (day of treatment) | 1 | 3 | NR | NR | NR |
| Lopinavir/Ritonavir (day of treatment) | 1 | 3 | 7 | 9 | NR |
| Methylprednisolone (day of treatment) | NR | 1 | 5 (stopped) | Reintroduced | 14 |
| Respiratory support | HFCN and NIV | NIV | NC | HFNC (3 d) | NR |
Abbreviations: CRP, C‐reactive protein; HFCN, high flow nasal cannula; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; NC, oxygen nasal cannula; ND, not done; NIV, noninvasive ventilation; NR, not received; PCT, procalcitonin; RT‐PCR SARS‐CoV‐2, reverse transcription‐polymerase chain reaction severe acute respiratory syndrome coronavirus 2; WBC, white blood cell count.
Forty‐eight hours later, he became apyretic with improved laboratory tests. However, he remained oxygen dependent on NIV. Blood culture taken in the ED was positive for S. pneumoniae. A new CXR showed increased pleural effusion, confirmed with chest ultrasound. A chest tube was placed (Figure 1) which drained fluid consistent with empyema. S. pneumoniae (immunochromatographic rapid test) and SARS‐CoV‐2 (RT‐PCR) were positive. Clindamycin (40 mg/kg/d/8 h) and steroids were started (methylprednisolone 1 mg/kg/d).
Clinical status progressively improved and he was weaned to HFNC 2 days later. On the 6th day of admission, he was switched to oxygen nasal cannula. A chest ultrasound showed significant improvement, so pleural tube was removed. He was discharged to the pediatric ward after 7 days in PICU. According to clinical improvement, oxygen supplementation and steroids were stopped on day 8.
Twenty‐four hours later, he developed fever, tachycardia, and moderate respiratory distress. The patient was readmitted to PICU, HFNC was started again. The RT‐PCR on NPS was repeated and still positive for SARS‐CoV‐2. A new CXR showed an air‐fluid level image in the LLL, consistent with necrotizing pneumonia, confirmed by chest computed‐tomography (Figure 1). New laboratory findings are described in Table 1. Also, new blood cultures were done to discard other bacteria or coinfections. Methylprednisolone and hydroxychloroquine were reinitiated. Antibiotic therapy was switched to intravenous meropenem (20 mg/kg/d/8 h) and linezolid (10 mg/kg/12 h) to broaden the antibiotic spectrum.
The patient improved during the next days. He was discharged to the pediatric ward again after 5 days in PICU. His chest ultrasound and bloodwork kept improving (Table 1). He received steroids for 14 days. He was discharged home after 18 days. At home, he continued oral linezolid to complete a total of 14 days and steroids on a tapering schedule for 4 more days up to 18 days. At the present, he is being followed as outpatient in the general pediatric clinics and his RT‐PCR for SARS‐CoV‐19 is already negative.
To our knowledge, this is the first case reporting a severe infant with SARS‐CoV‐2 and S. pneumoniae coinfection and SARS‐CoV‐2 detection in pleural effusion. According to a Chinese study, coinfection of SARS‐CoV‐2 and other virus is relatively common. Despite this, they did not completely investigate its clinical implications. 3 A preliminary Italian multicenter study by the Pediatric Infection Study Group describes the epidemiological, clinical, and therapeutic aspects of SARS‐CoV‐2 infection in children. It included 168 children positive for SARS‐CoV‐2, 65% required hospital admission and only two of them with previous comorbidities required intensive care admission and mechanical ventilation support. Ten children (5.9%) presented coinfections: nine with other viruses and one with a S. pneumoniae not as severe as the case described in this report. Clinical findings and specific treatment are not detailed. 2 The interest of our case lies in the simultaneous infection of SARS‐CoV‐2 and S. pneumoniae as cause of severe pneumonia with SARS‐CoV‐2 isolation in pleural effusion. 4 , 5 The possibility of antibiotic resistant bacteria as cause of necrotizing pneumonia was considered. It was studied and discarded through new blood cultures. Previous to this result and because clinical worsening, antibiotherapy was switched to meropenem and linezolid to modify the antimicrobial spectrum.
The clinical worsening after steroids removal could suggest a possible effectiveness of this therapy in the management of severe bacterial pneumonia and COVID‐19. Steroids are not usually part of the therapy for bacterial pneumonia due to the potential immunosuppressant effect. However, they could play a role in severe COVID‐19. The cytokines profile has been described similar to secondary hemophagocytic lymphohistiocytosis. 4 In regard to these findings along with the increased inflammatory markers in our case (Table 1), methylprednisolone was used. The steroids could treat the immune dysregulation produced by this virus. The efficacy of the empirical treatment given for COVID‐19 (azithromycin, lopinavir/ritonavir, and hydroxychloroquine) is uncertain.
In summary, we describe a severe case of SARS‐CoV‐2 and S. pneumoniae coinfection in a child. The patient was admitted in PICU with severe respiratory distress. He developed pleural effusion and, later, necrotizing pneumonia. The SARS‐CoV‐2 was positive in this fluid and this has not been previously described. There is still few information about the optimal management of COVID‐19 in children and the clinical consequences of SARS‐CoV‐2 coinfections with other microorganisms should be documented.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Work performed in Hospital Infantil Universitario Niño Jesús, Avenida Menéndez, Madrid, Spain.
REFERENCES
- 1. Zimmermann P, Curtis N. Coronavirus infections in children including COVID‐19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(18):2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin D, Liu L, Zhang M, et al. Co‐infections of SARS‐CoV‐2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020;63:606‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blanco‐Iglesias E, Oñoro G, Almodovar‐Martín JL, et al. Retrospective study in children with necrotizing pneumonia: nine years of intensive care experience. Pediatr Infect Dis J. 2020;39:571‐575. [DOI] [PubMed] [Google Scholar]


