Abstract
Data on the serological response toward severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 16 recent reports were analyzed and a high degree of variability was shown. Immunoglobulin M (IgM) responses were either found earlier than IgG, or together with IgG, later than IgG, or were missing. Therefore, clear distinctions between early, intermediate, and past infections are obviously not possible merely on the basis of IgM and IgG determinations. A review of publications on the serology of other virus groups shows that variable IgM responses can be found as well and therefore are not unique for SARS‐CoV‐2 infections. A model to explain this variability is proposed. The inclusion of avidity determination into regular diagnostic procedures has allowed to resolve such “atypical” serological constellations. The potential use of avidity determination for the diagnosis of COVID‐19, for risk assessment, epidemiological studies, analysis of cross reactions, as well as for the control of vaccination programs is suggested and discussed.
Keywords: affinity, avidity, IgG, SARS‐CoV‐2, serology, variability
Highlights
The serological response to SARS CoV‐2 infection is highly variable.
The mere detection of specific IgM and IgG does not allow to distinguish between acute and past infection.
The variable IgM and IgG responses after SARS CoV‐2 infection are analogous to serological findings in other virus systems.
Variable IgM and IgG responses can be rationally explained by models that describe immunoglobulin production by the immune system.
Avidity determination of SARS CoV‐2 IgG is suggested for resolution of diagnostic ambiguity.
1. INTRODUCTION
Severe acute respiratory syndrome corona virus‐2 (SARS‐CoV‐2) is presently causing a pandemic with many cases of severe disease (COVID‐19) and death. This has a massive impact on daily life, the health system, economy, politics, science, education, and international travel. Worldwide, governments and non‐government organizations try to develop strategies to counteract the pandemic and its consequences.
The management of COVID‐19 requires tools to
-
i)
diagnose or exclude SARS‐CoV‐2 infections in patients with respiratory symptoms;
-
ii)
define clinically asymptomatic as well as symptomatic persons who are infected with SARS‐CoV‐2, to prevent further spreading of the virus;
-
iii)
define persons who are seronegative for SARS‐CoV‐2 and therefore at risk for future SARS‐CoV‐2 infection;
-
iv)
define people with clinically asymptomatic SARS‐CoV‐2 infection and a positive immune response. It has to be clarified whether these people are protected towards reinfection by SARS‐CoV‐2 and how long this possible protection lasts.
There is an evolving consent that the detection of viral genomes through polymerase chain reaction (PCR), as well as the determination of specific antibody responses, will be required to answer the questions summarized above. Due to the characteristics of the viral infection and the resultant serological response, obviously none of these two approaches alone is sufficient for satisfactory diagnosis. It has already been shown that a higher degree of sensitivity for detection of SARS‐CoV‐2 infections is reached through a combination of PCR and antibody tests. 1 , 2 , 3 Thereby, the sensitivity of PCR alone was higher at the early phase of disease, whereas antibody tests alone were more favorable at later time points.
Based on its high specificity and sensitivity, PCR‐based detection of viral genomes has been proven as a valuable tool to determine SARS‐CoV‐2 replication in symptomatic, as well as asymptomatic‐infected persons. The PCR approach can clearly demonstrate infection activity, coinciding with recent contagion and acute illness in a certain number of cases. Importantly, a negative PCR result does not exclude SARS‐CoV‐2 infection, as the sample might have been taken too early or too late after infection. Obviously, the PCR technique is not suitable to determine individuals with past SARS‐CoV‐2 infection, as soon as these individuals do no longer shed virus.
For these reasons, there was a call for the development of test systems for specific detection of antibodies directed toward SARS‐CoV‐2. 4 , 5 The primary concept for developing these antibody tests was certainly not to substitute for PCR technology, but rather to complement it. It was suggested to use antibody tests for
-
i)
the confirmatory analysis of clinically apparent SARS‐CoV‐2 infections,
-
ii)
the detection of persons that had undergone clinically inapparent SARS‐CoV‐2 infection,
-
iii)
monitoring the success of immunization in the future.
The antibody response to SARS‐CoV‐2 infection seems to evolve after the onset of clinical symptoms 2 , 6 and after the beginning of virus replication and shedding. 7 Therefore, the absence of specific antibodies cannot exclude active SARS‐CoV‐2 infection, as the antibody response might not yet have been sufficiently developed. In such cases, additional testing at a later time point is required for clarification. This strategy bears the chance to eventually determine seroconversions. People without clinical symptoms, but with specific positive antibody results for SARS‐CoV‐2, can be concluded to have undergone infection with SARS‐CoV‐2. In analogy to other virus systems, they might have a good chance to be protected toward renewed SARS‐CoV‐2 infection and disease, but this issue needs further clarification. Antibody tests are important for epidemiological studies and for risk assessment. More data on the time period and extent of virus shedding after infection, as well as on the scale and quality of the subsequent immunological response are required. The scientific community is also aware of the necessity to avoid misinterpretations due to the detection of potentially cross‐reacting antibodies directed toward seasonal corona viruses in the SARS‐CoV‐2 antibody test systems.
The initial scientific discussion on the interpretation of antibody test results was usually based on a conventional text book view, which assumed that measurable immunoglobulin M (IgM) toward SARS‐CoV‐2 should always be detectable first during acute infection, whereas the detection of IgG was expected in a delayed mode. This view is derived from the clearly established order of steps during the initial phase of an immune response, where IgM‐generating B cells are subject to immunoglobulin class switch, resulting in IgG‐generating B cells. However, this strict sequence is not necessarily mirrored in the sequence of detectability of free IgM and IgG in the serum, as there are many additional steps between immunoglobulin class switch and the synthesis and appearance of free immunoglobulins in the serum.
The “classical view” on IgM responses preceding IgG responses has been encouraged by one of the first publications on SARS‐CoV‐2 serology, in which five of seven cases of acute infections with SARS‐CoV‐2 were serologically tested, using recombinant nucleoprotein and the enzyme‐linked immunosorbent assay (ELISA) technique. 8 Four cases were only tested on day 18 after the onset of clinical symptoms. Two of them were found positive for IgM and IgG toward SARS‐CoV‐2, whereas two were only positive for IgG. One additional case was serologically tested at different time points, starting on day 7 after onset of clinical symptoms. It showed an early seroconversion of IgM on day 9 after onset of clinical symptoms, with a later decline of IgM. This response was followed by seroconversion of IgG after week 2. The kinetics of the IgM and IgG responses of this particular case were then generalized. 4 , 6 However, later work by other authors showed that this “typical” kinetics seems to be the exception, as will be shown and discussed in more detail in this review.
As soon as more data on SARS‐CoV‐2 serology were available, it was evident that it is much more complex than initially anticipated. 2 , 5 , 9 For the practically acting expert, these findings were not surprising, as they were not different from the variable antibody responses seen after other viral infections.
This critical review therefore analyzes and tries to explain the variability of antibody responses toward SARS‐CoV‐2 infection measured in recently published manuscripts and preprints. In addition, a test regimen based on additional avidity determination is proposed, allowing to cope with “atypical” IgM responses.
1.1. The variability of the serological response to SARS‐CoV‐2 infection
Pan et al, 2 using a commercially available colloidal gold‐based immunochromatographic strip targeting IgM and IgG directed toward SARS‐CoV‐2 (antigens not disclosed) showed that the serological response to SARS‐CoV‐2 in PCR‐confirmed cases was delayed. During the first week after onset of symptoms, only 11% of the patients had a detectable immunological response, with IgM prevailing. In the period between 8 and 14 days, seropositivity increased to 78.6% for IgM, and 57% for IgG. At time points later than 15 days after onset of symptoms, IgM positivity remained at 74.8%, whereas positivity for IgG was rising to 96.8%. These findings indicate that serodiagnosis based on IgM alone would miss a substantial percentage of cases. The data also show that positivity for IgG was low early after onset of symptoms, but high at later time points. Nevertheless, in individual cases, the presence of IgG could not determine whether the infection was acute or past. The study by Pan et al 2 also showed that 43.6% of PCR‐negative cases with clinical symptoms for SARS‐CoV‐2 were found positive for antibodies toward SARS‐CoV‐2. The discrepancy between the PCR‐ and antibody‐based results might indicate that the time point of taking samples for PCR had been either too late, or that sampling or other critical steps had not been efficient. These findings underline the value of additional antibody determination. Cases with clinical symptoms, but with negative PCR and negative antibody results, might either have been caused by other agents than SARS‐CoV‐2, or might have represented very early cases.
The study by Long et al, 9 using a commercially available magnetic chemoluminescence enzyme immunoassay with recombinant nucleoprotein and a peptide from the spike protein of SARS‐CoV‐2, confirmed that seroconversion of IgM/IgG toward SARS‐CoV‐2 occurred after the onset of clinical symptoms and was preceded by positive PCR results. The median for seroconversion was 13 days after onset of disease. Importantly, this study demonstrated that the simplified picture of preceding and declining IgM, followed by late IgG, seemed not to be generally applicable to SARS‐CoV‐2 infection. In only 7 of 26 PCR‐positive cases with clinical symptoms, seroconversion of IgM preceded seroconversion of IgG. Nine cases showed synchronous seroconversion of IgM and IgG, whereas in 10 cases IgM converted to positivity even later than IgG.
These findings by Long et al 9 are summarized in Figure 1A. They illustrate i) that IgG responses are not necessarily confined to a later stage after infection, but even may be observed earlier than IgM; ii) that IgM responses may remain undetectable in the very early phase of infection, even if IgG is already detectable. Therefore, approaches to use the differentiation between IgM and IgG for an estimation of the time point relative to infection or the onset of clinical symptoms must obviously fail.
Figure 1.
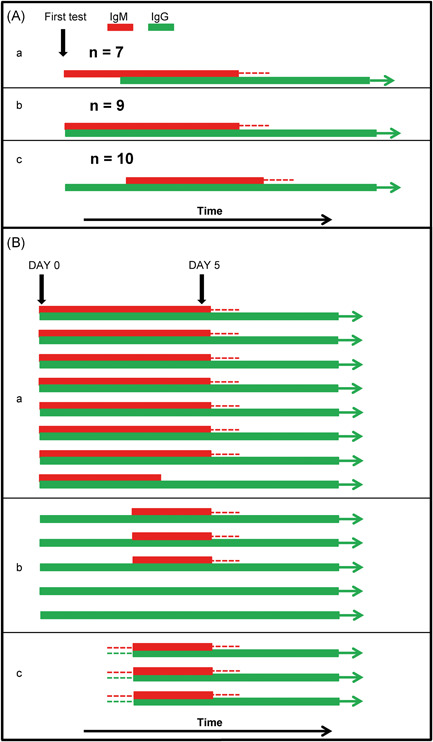
Variability of the serological response to SARS‐CoV‐2 infection. A, Schematic presentation of key findings presented in Long et al. 9 The analysis of the variability of the serological response in 26 cases of polymerase chain reaction (PCR) confirmed cases of SARS‐CoV‐2 infection shows that in seven cases immunoglobulin M (IgM) preceded IgG (a), in nine cases IgM and IgG seroconversion was determined at the same time, (b) and in 10 cases the IgM response was detected after the IgG response (c). B, Schematic presentation of key findings presented in Zhang et al. 5 The serological response in16 PCR‐confirmed cases of SARS‐CoV‐2 infections was analyzed on day 0 and day 5 after the onset of clinical symptoms. Group a: Eight cases showed positivity for IgM and IgG in parallel on day 0. Parallel positivity of IgG and IgM was maintained on day 5 except for one serum. Group b: Five cases showed IgG, but not IgM on day 0. In three of these cases, the IgM response was detectable on day 5 (“delayed IgM response”). In two cases, no IgM response was detectable at day 5, but acute infection was confirmed by the increase in the IgG response between day 0 and day 5. Group c: In three cases with confirmed infection, no serological response was detectable on day 0. Serum conversion both for IgM and IgG was detectable on day 5. The data from A and B demonstrate the high variability of the IgM and IgG response after acute SARS‐CoV‐2 infection. The number of cases with parallel or delayed IgM response (A) and the number of cases with parallel, delayed, negative IgM response (B) compared to the number of expected cases with preceding IgM (according to an outdated classical view) is highly significant (P = .0079 for A, .0005 for B, determined by the Yates continuity corrected χ 2 test) [Color figure can be viewed at wileyonlinelibrary.com]
The high variability of the patterns of IgM and IgG seroconversions related to SARS‐CoV‐2 was also demonstrated by Zhang et al, 5 who used an in house ELISA with SARSr‐CoV Rp3 nucleoprotein for the determination of IgM and IgG.
In this study, none of 16 patients with clinical symptoms and confirmed positive PCR for SARS‐CoV‐2, showed seroconversion of IgM before seroconversion of IgG. Eight patients showed simultaneous IgM and IgG towards SARS‐CoV‐2 on day zero. Thereby, in 7 of 8 cases the optical density for determined IgG was higher than for IgM. On day 5, seven cases remained positive for IgM and IgG, whereas in one case IgM turned out to be negative. In 5 cases of 16 patients, IgG was positive on day zero, while IgM was negative. On day 5, in three of five cases, IgM converted in a delayed mode, while the previously already positive IgG was rising to higher values. This rise of IgG confirms the significance of the finding, despite the delayed expression of IgM. In two cases, IgM remained negative also on day 5, while IgG was rising and thus confirmed the acute phase of infection. These two cases represent cases of acute infection without detectable IgM, demonstrating that a negative IgM result does not allow to exclude acute infection. Finally, this study comprised three cases that were seronegative for SARS‐CoV‐2 on day zero, but showed parallel seroconversion for IgM and IgG on day 5. Basic findings from the study by Zhang et al 5 are summarized in Figure 1B. They demonstrate that neither presence nor absence of IgM in the presence of IgG allows to differentiate between a very early or a later stage after infection and onset of clinical symptoms. Especially, the cases of seroconversion teach us that IgM may become detectable in parallel to IgG, or even after IgG, or may remain undetectable in few cases.
The study by Wölfel et al (using in house immunofluorescence tests with recombinant spike proteins of SARS‐CoV‐2) described the findings for nine SARS‐CoV‐2‐infected persons with mild clinical symptoms. 7 It was shown that seroconversion in 50% of patients occurred by day 7, and was completed in all patients at day 14. Seroconversion of IgM and IgG occurred without significant difference in time. Therefore, the results obtained by Wölfel et al 7 resemble the data obtained in the largest subgroup studied by Zhang et al 5 (Figure 1B, subgroup a). In analogy to Zhang et al, 5 Wölfel et al 7 showed that at days 12 to 28, IgM turned to negative in one case and to a borderline result in two cases. As Wölfel et al 7 were obviously expecting IgM preceding IgG, they were surprised by the parallel detection of IgM and IgG during seroconversion. The authors therefore speculated on competition between IgG and IgM for binding sites as underlying reason for their result. However, their assumption was not verified to be correct, as the time pattern of the IgM and IgG response was not changed when IgG had been removed from the sera before testing. Therefore, the findings by Wölfel et al 7 confirm the parallel detectability of IgM and IgG at seroconversion.
In the studies by Padoan et al 10 and Lippi et al, 11 the commercially available magnetic chemoluminescence enzyme immunoassay (MAGLUMI) with SARS‐CoV‐2 nucleoprotein and spike protein were used to study 87 serum samples from 37 patients 10 and samples from 48 patients. 11 Padoan et al detected the first seroconversions at 6 to 7 days after onset of clinical symptoms, whereas Lippi et al detected a small number of seroconversions (1 of 30 for IgM, 3 of 30 for IgG) already during the first 5 days. In both studies, the phenomenon of delayed appearance of IgM, as well as missing IgM despite the presence of IgG was observed in a significant number of cases. Thereby, delayed and missing IgM responses were found more frequently in a study by Lippi et al. 11
Liu et al, 3 using PCR and a commercially available ELISA with viral nucleoprotein reported on the results obtained for 283 confirmed patients. They concluded that before day 11 after the onset of symptoms, PCR is the method of choice, whereas after 11 days the determination of antibodies is advisable. Seroconversion in most cases was at day 7 and later, with most cases showing IgM and IgG toward SARS‐CoV‐2 in parallel, and only few cases with either isolated IgM or IgG. These data confirm the high degree of variability of the serological response, as well as the frequent parallel appearance of IgM and IgG.
Verdoni et al 12 report on the findings on 10 children (2.9‐16 years) with suspected SARS‐CoV‐2‐associated Kawasaki syndrome. Two children were seronegative for SARS‐CoV‐2 antibodies, whereas five showed IgG in the absence of IgM, and three showed IgM plus IgG.
Hoffman et al 13 report on the study of 29 PCR‐confirmed cases of SARS‐CoV‐2 infection, using a commercially available rapid test cassette (without disclosure of antigens). Sera from 20/29 patients showed IgM toward SARS‐CoV‐2 and 27/29 contained IgG toward the virus. All IgM‐positive cases were also positive for IgG. These findings confirm the absence of IgM in a substantial percentage of cases, either through delayed or missing appearance.
Whereas Xiao et al 14 (CLIA with nucleoprotein and S protein; 34 patients), Liu et al 15 (commercial ELISA with nucleoprotein and S protein) and To et al 16 (commercial ELISA, nucleoprotein and S protein, 23 patients) observed no significant difference between the kinetics of seroconversion for IgM and IgG toward SARS‐CoV‐2, Jin et al 17 (CLIA, nucleoprotein and S protein, 43 cases) and Qu et al 18 (CLIA, nucleoprotein and S protein, 347 sera from 41 patients) reported on a delayed appearance of IgM compared with IgG.
Xiang et al 19 (using a commercial ELISA, nucleoprotein, 216 samples from 85 patients) reported on a slightly higher cumulative seroconversion rate for IgM compared with IgG. Similarly, Zhao et al 1 (analyzing 535 serum samples from 173 patients, using a µ‐capture ELISA with recombinant receptor binding domain of S protein for IgM determination and a ELISA with recombinant nucleoprotein for the determination of IgG) reported a higher seroconversion rate for IgM, as well as an earlier median seroconversion time of IgM (12 days) compared with IgG (14 days). However, the kinetic analysis of the antibody dynamics of 9 selected cases revealed a high degree of variability. IgM was preceding IgG in 2 of 9 cases, IgM and IgG appeared at the same time in 3 of 9 cases, a delayed IgM response as well as missing IgM in the presence of IgG were found in 1 of 9 cases each. In two cases, both IgM and IgG only reached background activity. The limitation of the study by Zhao et al 1 is the use of different antigens for the determination of IgM and IgG, respectively.
The studies on IgM and IgG responses toward SARS‐CoV‐2 discussed above show a high degree of variability, independent of the methods and the antigens used for detection, confirming that the problem of variability of the immune response is real.
The significance of these conclusions is also supported by data obtained for SARS‐CoV‐1 in 2003, where a low sensitivity of IgM determinations had also been noted. 20 Furthermore, Li et al 21 showed that out of 20 patients with confirmed SARS‐CoV‐1 infection, none had a preceding IgM response, but serum conversion for IgM and IgG seemed to occur in parallel. In addition, Hsueh et al 22 showed delayed IgM responses compared with IgG after acute SARS‐CoV‐1 infections, as well as a persistent IgM response in past infection.
In summary, the determination of specific IgM and IgG responses allows to define seropositivity for SARS‐CoV‐2, but does not allow to determine the time point in relation to infection or the onset of clinical symptoms. This is due to the high variability of the IgM response. Despite its usually limited presence during the acute phase of infection, IgM may appear before IgG, together with IgG or even after IgG. IgM may even be missing during acute infection in a certain number of cases. In analogy to the situation found for SARS‐CoV‐1, it is not unlikely that IgM toward SARS‐CoV‐2 might also persist for longer times in rare cases.
1.2. Variability of the serological response to other viral infections
The high variability of IgM and IgG responses toward SARS‐CoV‐2, as summarized in this review, is not unique for infections with this virus. High variability of IgM and IgG responses rather seems to be a regularly occurring phenomenon, though the frequency and impact of this phenomenon are often underestimated. There are a few examples of IgM preceding the IgG response in a substantial percentage of cases (as shown for hepatitis E acute infections 23 ), but many examples of parallel detectability of IgM and IgG, such as acute infections by hepatitis C, 24 EBV (reviewed by De Pascale and Clerici 25 ) and SARS‐CoV‐1. 21 In practice, parallel detection of IgM and IgG seems to represent the most prominent situation in routine serology of many viral systems.
Delayed IgM responses, that is, IgG occurring before IgM during acute infection, have been found for the serology of enteroviruses, 26 HSV, 27 measles virus, 28 EBV 29 and HCV. 30
IgM missing in a certain percentage of cases during acute infections has been reported for parvovirus B 19, 31 HAV, 32 EBV, 29 Poliomyelitis virus, 33 measles virus, 34 Japanese encephalitis virus, 35 tick‐borne encephalitis virus, 36 rubella virus, 37 HCV, 30 and CMV. 38
Whereas missing or apparently late appearing IgM responses may have caused misdiagnosis of acute infections as past infections, the opposite problem was arising through the detection of persistently positive IgM responses for time periods of many months or even few years, as found for borrelia infection, 39 rubella virus, 40 , 41 EBV, 29 Puumala virus, 42 parvovirus B 19, 43 HCV, 30 and TBE virus. 44
The high degree of variability of the kinetics of the IgM response, as well as problems like misleading IgM responses after polyclonal stimulation by certain agents have been recognized as a severe problem in serology, as reviewed by Landry. 45
How can the high variability of the kinetics of IgM and IgG responses after viral infections be explained? Figures 2 and 3 summarize the essential steps between the induction of an immune response and the appearance of IgM and IgG in serum. The figures show that simple changes in the time of immunoglobulin isotype switching and differentiation of IgM‐positive or IgG‐positive B cells to plasma cells are sufficient to explain the observed patterns of variable IgM/IgG responses. In addition, differences in the half‐life of IgM and IgG, differences in the sensitivities of the test system, as well as competition between IgM and IgG for the target structures can be predicted to have a profound influence on the final outcome of IgG and IgM determination in the serum.
Figure 2.
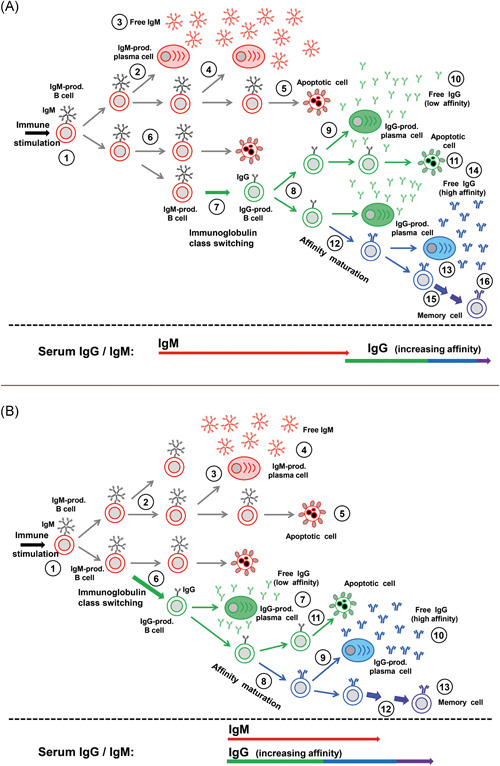
Variability of the immunoglobulin M (IgM) response: preceding IgM and parallel appearance of IgM and IgG A, “Classical picture” with IgM preceding IgG. An immune stimulus generates B cells that generate IgM toward a defined epitope (#1). Replication of this cell can lead to the generation of plasma cells and the massive production of free IgM (#2‐4), to apoptotic cell death (#5, 6) or to immunoglobulin class switch and the generation of IgG‐producing B cells (#7). These may either differentiate into plasma cells and generate low affinity IgG (#8‐10), die through apoptosis (#11) or contribute to the generation of IgG of higher affinity through clonal selection (#12‐14). The increase in affinity/avidity occurs in several steps (#15) and leads to the generation of memory cells (#16) that allow anamnestic responses. The setting shown in A leads to the classical picture of IgM preceding IgG in the serum. B, Parallel occurrence of IgM and IgG in the serum. Assuming that IgM‐producing plasma cells are generated later than shown under A (#1‐3), and the immunoglobulin class switch (#6) is occurring earlier than in the scenario described under A, the production of IgM and IgG through plasma cells (#3, #7) will occur in parallel and lead to the parallel detectability of IgM and IgG in the serum—despite the fact that IgM‐presenting B cells had been established before IgG‐presenting B cells [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
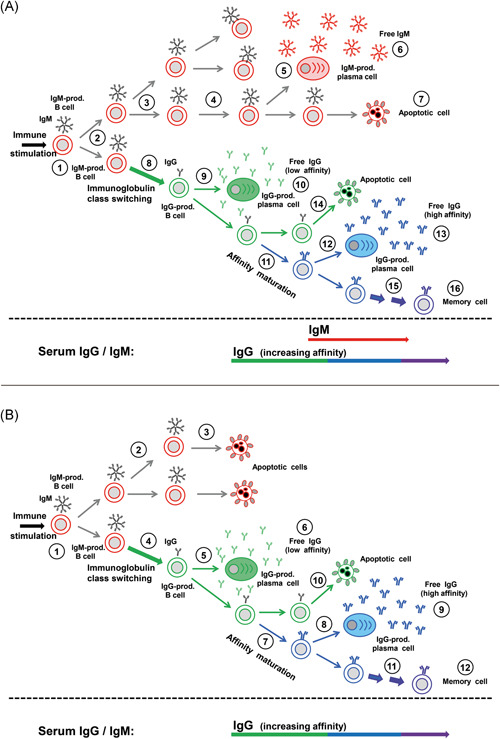
Variability of the immunoglobulin M (IgM) response: delayed and missing IgM responses. A, Delayed IgM response. A delayed differentiation of IgM‐presenting B cells to plasma cells (#1‐5), in combination with a fast immunoglobulin class switch (#8) and generation of IgG‐producing plasma cells (#9) explains the frequently observed phenomenon of delayed appearance of IgM in the serum, though IgM‐presenting B cells are always generated first. B, Missing IgM response in the serum. A low degree of replication of IgM‐presenting B cells and their early cell death (#3) prevents differentiation to IgM‐producing plasma cells and does not lead to detectable IgM in the serum, whereas IgG is produced and matures with respect to avidity. This scenario explains how IgG production depends on IgM‐presenting B cells, without IgM being detectable in the serum [Color figure can be viewed at wileyonlinelibrary.com]
1.3. How was the problem of variable IgM responses satisfactorily resolved to reach a rational and unambiguous diagnostics?
Especially, the pioneering work of Hedman and coworkers led to the establishment of quantitative measurements of IgG avidity and thus allowed for a clear distinction between acute infection with IgG of low avidity and past infection with high avidity of IgG. 37 , 46 The discussion of this useful method requires a quick reflection about the underlying biological mechanisms: During an immune response, clonal selection of B cells that generate IgG with higher affinity leads to the gradual increase in affinity of serum IgG. 47 , 48 , 49 , 50 Affinity of IgG toward an antigen represents a complex biophysical process, determined by the binding and the release reaction of antibody from its target. As this process cannot be easily measured in routine applications, applied research restricted the analysis on determining the strength of the binding between antigen and IgG. This was measured by allowing first an undisturbed antibody‐antigen reaction, and then testing the degree of release of antibody through the application of chaotropic agents like urea. This approach has been termed “avidity” determination (derived from latin aviditas, meaning desire, greed) or determination of “functional affinity”. (It is noteworthy that the term “avidity” as used in modern serology is defining a different biochemical setting than the same word previously used to describe the complexity of multivalent antibody and target structure populations. The use of one term for two different aspects frequently leads to confusion.)
The determination of avidity starts with the incubation of serum with the test system in duplicate. After a washing step, one of the assays is immediately processed to quantification of bound IgG, whereas the parallel assay is first treated with defined concentrations of urea. After a subsequent washing step, this test is processed for quantification of bound IgG. By comparing the results between the urea‐treated and the control assay, an avidity index can be determined and used for differentiation between acute, intermediate, and past infections.
Avidity determination is now used as a valuable analytical method in many diagnostic laboratories. It is one of the key elements for the confirmation or disproval of acute rubella virus infection, particularly during pregnancy. 37 , 46 Avidity determination is instrumental to resolve the complex serology of human herpesviruses, such as EBV, 25 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 CMV, 59 , 60 , 61 , 62 HHV‐6, and HHV‐7. 63 It has been used for rational diagnostics of tick‐borne encephalitis virus, 36 parvovirus B 19, 64 measles virus, 65 , 66 , 67 mumps virus, 68 West nile virus, 69 , 70 hepatitis A virus, 71 hepatitis C virus, 72 , 73 , 74 hepatitis E virus, 23 Dengue virus, 75 Borrelia, 76 and toxoxplasmosis. 77
Avidity determination can be used to differentiate between primary immune responses (with initial low avidity and further avidity maturation) and anamnestic responses, established by memory B cells that generate IgG of high avidity. 65 , 66 , 68
In summary, IgG avidity determination allows a clear differentiation between acute and past infection, as affinity maturation is a regular feature of long‐lasting immune responses. Furthermore, affinity maturation is unidirectional and therefore irreversible. Therefore, avidity determination has the potential to unequivocally differentiate between acute, intermediate, and past infection, irrespective of the pattern of the IgM responses (Figure 4).
Figure 4.
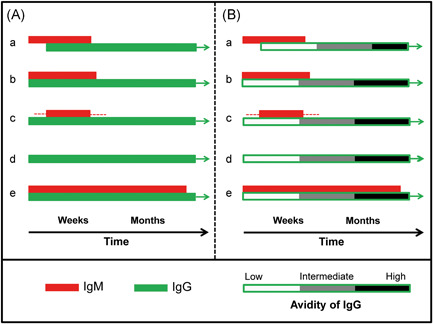
Proposed resolution of serological ambiguity through determination of avidity (functional affinity). A, Possible serological immunoglobulin M (IgM)/IgG constellations following primary viral infection are schematically summarized. In serological practice, the “text book constellation” with IgM seroconversion preceding IgG seroconversion (a) is rather rare. Parallel determination of IgM and IgG usually represents the most frequent case (b). Delayed occurrence of IgM is less frequent (c). In several cases of acute infections, the detection of the IgM may be missed (d). This may be due to low expression of IgM response, problems of sensitivity of the assay used, competition of IgM by IgG during the assay or missing the right time point of positivity of the response. In few cases, IgM responses may persist for longer times after primary infection (e). As discussed in the text, constellations a‐d have been reported for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections. Based on the experience with SARS‐CoV‐1 and many other viral systems, constellation "e" can be predicted to occur as well and may be detected as soon as longer follow‐up studies will have been performed. Part A shows that the determination of IgM and IgG does not allow to draw an unequivocal conclusion on the time point of infection or beginning of clinical symptoms. B, The inclusion of IgG avidity allows an unambiguous determination of early, intermediate, and past infection, irrespective of the variability of the IgM response. The diagnostic power of avidity determination has been shown for many viral systems. It is suggested to include this general immunological feature into routine diagnostics of SARS‐CoV‐2 infections [Color figure can be viewed at wileyonlinelibrary.com]
1.4. Proposal to use avidity determination for the serological diagnosis of SARS‐CoV‐2 infection
As SARS‐CoV‐2 serology is characterized by high variability, and as avidity determination seems to be the key method to resolve analogous problems in other viral systems, we propose to establish and evaluate avidity determination of SARS‐CoV‐2 IgG in the near future. This approach has a predictable potential to improve serodiagnosis of SARS‐CoV‐2 infections.
Establishment of SARS‐CoV‐2 avidity testing requires a specific and quantitative test system. The precise interpretation of results will require to determine the kinetics of affinity maturation of IgG directed toward SARS‐CoV‐2 and to determine the frequency of patients possibly showing a failure in affinity maturation. The determination of the kinetics of avidity maturation requires to use sufficiently high concentrations of antigen, as limited antigen concentrations select against low avidity IgG in a mixture of IgG of different avidity and thus shorten the window of low avidity detection after acute infection. 58
The hope for successful establishment of SARS‐CoV‐2 avidity tests is based on rational optimism, as all basic requirements seem to be fulfilled for the related SARS‐CoV‐1: Chan et al 78 , 79 have carefully established and evaluated avidity determination for SARS‐CoV‐1. The kinetics of affinity maturation has been measured and no indication for a failure to finally reach high avidity was observed. Due to the biological and biochemical similarity between SARS‐CoV‐1 and SARS‐CoV‐2, the establishment of avidity testing of IgG directed toward SARS‐CoV‐2 therefore seems to have a realistic chance.
Based on the experimental and practical experience with many other viral systems, it is predicted that it should be possible to define four stages of the infection status through avidity determination of IgG toward SARS‐CoV‐2:
-
i)
Seronegativity for IgG toward SARS‐CoV‐2 in uninfected persons or infected persons very early in infection.
-
ii)
Low avidity IgG toward SARS‐CoV‐2 as strong indication for acute infection. Further, proof can be achieved through determination of subsequent avidity maturation.
-
iii)
Intermediate avidity IgG toward SARS‐CoV‐2 indicative for recent infection.
-
iv)
High avidity IgG toward SARS‐CoV‐2 as indication for past infection.
2. AVIDITY DETERMINATION FOR THE RESOLUTION OF PROBLEMS DUE TO CROSS REACTIVITY
Reactivity of antibodies induced by a defined virus and directed toward analogous proteins of a related viruses, that is classical cross reactivity of antibodies, is a well‐known phenomenon for corona viruses. Chan et al have shown that stored sera from patients, convalescent for SARS‐CoV‐1 infection, were reactive toward MERS‐CoV—a virus that had not been in the human population when the sera had been collected. 80 As sera from healthy controls from the same time did not react with MERS‐CoV, the dependence of cross reactivity on the antibodies originally induced by SARS‐CoV‐1 was ensured. This cross reactivity can be explained by common epitopes of analogous proteins of related viruses. It remains to be determined, whether this type of rather strong reactivity is caused by high avidity of the involved antibodies.
A more complex type of interaction between the viruses and the immune system has been reported by Chan et al. 79 They demonstrated that infection with SARS‐CoV‐1 (ensured by seroconversion) was accompanied by the appearance of low avidity antibodies, followed by avidity maturation after 1 month. In parallel, the concentration of high affinity antibodies directed toward seasonal coronaviruses (HuCoV‐OC43 and HuCoV‐229E) that were already present in the sera before seroconversion of SARS‐CoV‐1, increased with seroconversion. The power of avidity determination to discriminate between the de novo immune response toward SARS‐CoV‐1 (with its initially low avidity IgG) and the anamnestic response of antibodies (with immediate high avidity of IgG) directed toward related corona viruses is elegantly demonstrated in this experiment. Therefore, avidity determination applied to SARS‐CoV‐2 serology has a good chance to contribute to the resolution of complex problems associated with cross reactivity and interactions between different coronaviruses. These aspects are especially important, as many of the test systems used so far and reviewed in this manuscript show specificity in the range between 95% and 99%, which is still problematic with respect to the frequent occurrence of seasonal corona viruses and the relatively lower incidence of SARS‐CoV‐2 infection.
2.1. Avidity and the protective effect of antibodies
It has been established that long‐lasting protective humoral immunity requires the generation of high avidity IgG and memory B cells that can generate such antibodies upon adequate stimulation. 81 , 82 , 83 , 84 , 85 , 86 , 87 Therefore, the determination of the kinetics of IgG avidity maturation will be necessary for getting an estimation about the quality, as well as the duration of protection toward reinfection by SARS‐CoV‐2. The present discussion on reinfection with SARS‐CoV‐2 is positioned between the suggestions by Edridge et al, 88 who assume that the short protective period (<6‐12 months) determined for seasonal coronaviruses might be a model for an analogous situation for SARS‐CoV‐2, and Kellam and Barclay, 89 who suggest that the stronger viremia of SARS‐CoV‐2 compared to seasonal corona viruses should trigger a stronger immune response, characterized by finally high affinity/avidity. The conclusions by Kellam and Barclay 89 are in agreement with conclusions by Chan et al, 80 related to antibody‐mediated protection toward MERS CoV and SARS‐CoV‐2. The clarification of this open theme should gain from avidity determination in the future and provide further insights into the aspects of protection toward SARS‐CoV‐2. Based on established work in other viral systems, it is predictable that reinfections with SARS‐CoV‐2 due to low antibody concentrations and low avidity after primary infection would result in an immune response that started with low avidity and might develop avidity maturation, whereas reinfection due to low concentrations of high affinity antibodies would induce an anamnestic response with immediate increase of high avidity antibodies.
In line with these considerations and established work, 81 , 82 , 83 , 84 , 85 , 86 , 87 it is also predictable that avidity determination of IgG established through immunization with SARS‐CoV‐2 antigens in the future will be essential to ensure the quality of protection toward the virus. In line with the findings by Kontio et al, 86 the parallel measurement of antibody concentration and avidity might determine the hopeful success of future vaccination programs toward SARS‐CoV‐2.
3. CONCLUSIONS
The serological response after infection with SARS‐CoV‐2 is highly variable, showing IgM preceding IgG, occurring in parallel with IgG, appearing in a delayed mode compared to IgG, as well as missing IgM after acute infection. In analogy to the findings for many other virus groups and particularly for SARS‐CoV‐1, the establishment of avidity determination for SARS‐CoV‐2 infection is proposed to ensure unambiguous serological diagnosis. It is predicted that avidity determination might allow differentiation between cross‐reacting corona viruses, analysis of reinfections with SARS‐CoV‐2, and help to determine the quality of the immune status after future immunization programs.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
Corrections and suggestions made by Dr. Hans‐Helmut Niller (University of Regensburg, Germany), as well as technical support by Hans‐Jürgen Brandel (University of Freiburg) are gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
Bauer G. The variability of the serological response to SARS‐corona virus‐2: Potential resolution of ambiguity through determination of avidity (functional affinity). J Med Virol. 2021;93:311–322. 10.1002/jmv.26262
REFERENCES
- 1. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan Y, Li X, Yang G, et al. Serological immunochromatographic approach in diagnosis with SARS‐CoV‐2 infected COVID‐19 patients. medRxiv. 2020. 10.1101/2020.03.13.20035428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Liu W, Zheng Y, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 238 admitted hospital patients. Microb Infect. 10.1016/j.micinf.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao S‐Y, Wu Y, Liu H. Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol. 2020;92:464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Du R‐H, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386‐389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 7. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. 10.1038/s41586-020-2196-X [DOI] [PubMed] [Google Scholar]
- 8. Zhou P, Yang X, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long Q‐X, Deng H‐J, Chen J, et al. Antibody responses to SARS‐CoV‐2 in COVID‐19 patients: the perspective application of serological tests in clinical practice. medRxiv. 2020. 10.1101/2020.03.18.20038018 [DOI] [Google Scholar]
- 10. Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS‐CoV‐2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020;58:1081‐1088. 10.1515/cclm-2020-0443 [DOI] [PubMed] [Google Scholar]
- 11. Lippi G, Salvagno GL, Pegoraro M, et al. Assessment of immune response to SARS‐CoV‐2 with fully automated MAGLUMI 2019‐nCoV IgG and IgM chemiluminescence immunoassays. Clin Chem Lab Med. 2020;58:1156‐1159. 10.1515/cclm-2020-0473 [DOI] [PubMed] [Google Scholar]
- 12. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. The Lancet. 2020;395:1771‐1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman T, Nissen K, Krambrich J, et al. Evaluation of a COVID‐19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARSCoV‐2. Infect Ecol Epidemiol. 2020;10(1):1754538. 10.1080/20008686.2020.1754538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS‐CoV‐2: the first report. J Infect. 2020;81:147‐178. 10.1016/j.jinf.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu W, Liu L, Kou G, et al. Evaluation of nucleocapsid and spike protein‐based enzyme‐linked immunosorbent assays for detecting antibodies against SARS‐CoV‐2. J Clin Microbiol. 2020;58:e00461‐20. 10.1128/JCM.00461-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. To KK‐W, Tsang OT‐Y, Leung W‐S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20:565‐574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin Y, Wang M, Zuo Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease. Int J Infect Dis. 2020;94:49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qu J, Wu C, Li X, et al. Profile of immunoglobulin g and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. 10.1093/cid/ciaa489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo PCY, Lau SKP, Wong BHL, et al. Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia. J Clin Microbiol. 2004;42:2306‐2309. 10.1128/JCM.42.5.2306-2309.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li G, Chen X, Xu A. Profile of specific antibodies to the SARS‐associated coronavirus. N Engl J Med. 2003;349:508‐509. [DOI] [PubMed] [Google Scholar]
- 22. Hsueh P‐R, Huang L‐M, Chen P‐J, Kao C‐L, Yang P‐C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS‐associated coronavirus. Clin Microbiol Infect. 2004;10:1062‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bendall R, Ellis V, Ijaz S, Thurairajah P, Dalton HR. Serological response to hepatitis E virus genotype 3 infection: IgG quantitation, avidity and IgM response. J Med Virol. 2008;80:95‐101. [DOI] [PubMed] [Google Scholar]
- 24. Quiroga JA, Campillo ML, Catillo J, Bartolome J, Porres JC, Carreno V. IgM antibody to hepatitis C virus in acute and chronic hepatitis C. Hepatology. 1991;14:38‐43. [DOI] [PubMed] [Google Scholar]
- 25. De Paschale M, Clerici P. Serological diagnosis of Epstein‐Barr virus infection: problems and solutions. World J Virol. 2012;1:31‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glimaker M, Samuelson A, Magnius L, Ehrnst A, Olcen P, Forsgren M. Early diagnosis of enteroviral meningitis by detection of specific IgM antibodies with a solid phase reverse immunosorbent test (SPRIST) and mu‐capture EIA. J Med Virol. 1992;36:193‐201. [DOI] [PubMed] [Google Scholar]
- 27. Ho DW, Field PR, Sjogren Janson E, Jeansson S, Cunningham AL. Indirect ELISA for the detection of HSV‐2 specific IgG and IgM antibodies with glycoprotein G (gG‐2). J Virol Methods. 1992;36:249‐264. [DOI] [PubMed] [Google Scholar]
- 28. Hummel KB, Erdmann DD, Heath J, Bellini WJ. Baculoexpression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874‐2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schillinger M, Kampmann M, Henninger K, Murray G, Hanselmann I, Bauer G. Variability of humoral immune response to acute Epstein‐Barr virus (EBV) infection: evaluation of the significance of serological markers. Med Microbiol Lett. 1993;2:296‐303. [Google Scholar]
- 30. Zaaijer HL, Mimms LT, Cuypers HT, et al. Variability of the IgM response in hepatitis C virus infection. J Med Virol. 1995;40:184‐187. [DOI] [PubMed] [Google Scholar]
- 31. Al Frayh AR, Bakahim H, Kidess E, Ramia S. IgG and IgM antibodies to human parvovirus B19 in the serum of patients with a clinical diagnosis of infection with the virus and the general population in Saudi Arabia. J Infect Dis. 1993;27:51‐55. [DOI] [PubMed] [Google Scholar]
- 32. Brackmann HH, Oldenburg J, Eis‐Hübinger AM, Gerritzen A, Hammerstein U, Hanfland P. Hepatitis A virus infection among the hemophilia population at the Bonn Hemophilia Center. Vox Sang. 1994;67(suppl 1):3‐7. [PubMed] [Google Scholar]
- 33. Roivainen M, Agboatwalla M, Tenvik M, Rysa T, Akram DS, Hovi T. Intrathecal immune response and virus‐specific immunoglobulin M antibodies in laboratory diagnosis of acute poliomyelitis. J Clin Microbiol. 1993;31:2427‐2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaz de Lima LR, Hoshino‐Shimizu S, de Souza VA, et al. Measles serodiagnosis: standardization and evaluation of a Dot‐ELISA. Rev Inst Med Trop Sao Paulo. 1994;36:139‐147. [DOI] [PubMed] [Google Scholar]
- 35. Rathi AK, Kushwah KP, Singh YD, Soirohi R, Singh RK, Singh UK. JE virus encephalitis: 1988 epidemic at Gorakhpur. Indian Pediatr. 1993;30:325‐333. [PubMed] [Google Scholar]
- 36. Gassmann C, Bauer G. Avidity determination of IgG directed against tick‐borne encepahilits virus improves detection of current infections. J Med Virol. 1997;51:242‐251. [PubMed] [Google Scholar]
- 37. Hedman K, Rousseau SA. Measurement of avidity of specific IgG for verification of recent primary rubella. J Med Virol. 1989;27:288‐292. 10.1002/jmv.1890270406 [DOI] [PubMed] [Google Scholar]
- 38. Brytting M, Xu W, Wahren B, Sundqvist VA. Cytomegalovirus DNA detection in sera from patients with active cytomegalovirus infections. J Clin Microbiol. 1992;30:1937‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammers‐Berggren S, Hansen K, Lebach AM, Karlsson M. Borrelia burgdorferi‐specific intrathecal antibody production in neuroborreliosis: a follow‐up study. Neurology. 1993;43:169‐175. [DOI] [PubMed] [Google Scholar]
- 40. Thomas HI, Morgan‐Capner P, Roberts A, Hesketh L. Persistent rublla‐specific IgM reactivity in the absence of recent primary rubella infection and rubella reinfection. J Med Virol. 1992;36:188‐192. [DOI] [PubMed] [Google Scholar]
- 41. Hedman K, Seppalä I. Recent rubella infection indicated by a low avidity of specific IgG. J Clin Immunol. 1988;8:214‐221. [DOI] [PubMed] [Google Scholar]
- 42. Lundkvist A, Horling J, Niklasson B. The humoral response to Puumala virus infection (nephropatia epidemica) investigated by viral protein specific immunoassays. Arch Virol. 1993;130:121‐130. [DOI] [PubMed] [Google Scholar]
- 43. Erdmann DD, Usher MJ, Tsou C, et al. Human parvovirus B19 specific IgG, IgA, and IgM antibodies and DNA in specimens from persons with erythema infectiosum. J Med Virol. 1991;35:110‐115. [DOI] [PubMed] [Google Scholar]
- 44. Roggendorf M, Heinz F, Deinhardt F, Kunz C. Serological diagnosis of tick borne encephalitis by demonstration of antibodies of the IgM class. J Med Virol. 1981;7:41‐50. [DOI] [PubMed] [Google Scholar]
- 45. Landry ML. Immunoglobulin M for acute infection: true or false? Clin Vaccine Immunol. 2016;23:540‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hedman K, Lappalainen M, Söderlund M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1997;4:123‐129. [Google Scholar]
- 47. Eisen HN, Siskind GW. Variations in the affinities of antibodies during the immune response. Biochemistry. 1964;3:996‐1008. [DOI] [PubMed] [Google Scholar]
- 48. Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991;352:530‐531. [DOI] [PubMed] [Google Scholar]
- 49. Wolter T, Gassmann C, Vetter V, Bauer G. Avidity determination: utilization of a basic immunological mechanims allows to improve serological diagnosis of infections. Clin Lab. 1997;43:125‐135. [Google Scholar]
- 50. Hazel SL. Clinical utility of avidity assays. Expert Opin Med Diagnostics. 2007;1:511‐519. [DOI] [PubMed] [Google Scholar]
- 51. Andersson A, Vetter V, Kreutzer L, Bauer G. The avidities of IgG directed against viral capsid antigen (VCA) or early antigen (EA): useful markers for a more significant Epstein‐Barr virus serology. J Med Virol. 1994;43:238‐244. [DOI] [PubMed] [Google Scholar]
- 52. Vetter V, Kreutzer L, Bauer G. Differentiation of primary from secondary anti‐EBNA‐1‐negative cases by determination of avidity of VCA‐IgG. Clin Diag Virol. 1994;2:29‐39. [DOI] [PubMed] [Google Scholar]
- 53. Bauer G. Simplicity through complexity: immunoblots with recombinant antigens as the new gold standard in Epstein Barr virus serology. Clin Lab. 2001;47:223‐230. [PubMed] [Google Scholar]
- 54. Pottgiesser T, Wolfarth B, Schumacher YO, Bauer G. Epstein‐Barr virus serostatus: no difference despite aberrant patterns in athletes and control groups. Med Sci Sports Exerc. 2006;38:1782‐1791. [DOI] [PubMed] [Google Scholar]
- 55. Pottgiesser T, Schumacher YO, Wolfarth B, Schmidt‐Trucksäss A, Bauer G. Longitudinal observation of Epstein‐Barr virus antibodies in athletes and controls during a competition season. J Med Virol. 2012;84:1415‐1422. [DOI] [PubMed] [Google Scholar]
- 56. Schubert J, Zens W, Weissbrich B. Comparative evaluation of the use of immunoblots and of IgG avidity assays as confirmatory tests for the diagnosis of acute EBV infections. J Clin Virol. 1998;11:161‐172. 10.1016/S0928-0197(98)00061-0 [DOI] [PubMed] [Google Scholar]
- 57. Robertson P, Beynon S, Whybin R, et al. Measurement of EBV‐IgG anti‐VCA avidity aids the early and reliable diagnosis of primary EBV infection. J Med Virol. 2003;70:617‐623. [DOI] [PubMed] [Google Scholar]
- 58. Niller HH, Bauer G. Epstein‐Barr virus: clinical diagnostics. Meth Mol. Biol. 2017;1532:33‐55. [DOI] [PubMed] [Google Scholar]
- 59. Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15:680‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prince HE, Leber AL. Validation of an in‐house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin Diagn Lab Immunol. 2002;9:824‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lazzarotto T, Varani S, Spezzacatena P, et al. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Virol Immunol. 2009;13:137‐141. 10.1089/vim.2000.13.137 [DOI] [PubMed] [Google Scholar]
- 62. Bodéus M, Feyder S, Goubau P. Avidity of IgG antibodies distinguishes primary from non‐primary cytomegalovirus infection in pregnant women. Clin Diagn Virol. 1998;9(1):9‐16. 10.1016/S0928-0197(97)10016-2 [DOI] [PubMed] [Google Scholar]
- 63. Ward KN, Turner DJ, Couto Parada X, Thiruchelvam AD. Use of immunoglobulin G antibody avidity for differentiation of primary human herpesvirus 6 and 7 infections. J Clin Microbiol. 2001;39:959‐963. 10.1128/JCM.39.3.959-963.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Söderlund M, Brown C, Cohen BJ, Hedman K. Accurate serodiagnosis of B 19 parvovirus infections by measurement of IgG avidity. JID. 1995;171:710‐713. [DOI] [PubMed] [Google Scholar]
- 65. Paunio M, Hedman K, Davidkin I, Peltola H. IgG avidity to distinguish secondary from primary measles vaccination failures: prospects for a more effective global measles elimination strategy. Exp Opininion Pharmacotherapy. 2005;4:1215‐1225. [DOI] [PubMed] [Google Scholar]
- 66. Mercader S, Garcia P, Bellini WJ. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clin Vacc Immunol. 2012;19:1810‐1817. 10.1128/CVI.00406-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Narita M, Yamada S, Matsuzono Y, Itakaura O, Togashi T, Kikuta H. Immunoglobulin G avidity testing in serum and cerebrospinal fluid for analysis of measles virus infection. Clin Diagn Lab Immunol. 1996;3:211‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Narita M, Matsuzono Y, Takekoshi Y, et al. Analysis of mumps vaccine failure by means of avidity testing for mumps virus‐specific immunoglobulin G. Clin Diagn Lab Immunol. 1998;5:799‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Levett PN, Sonnenberg K, Sidaway F, et al. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with west nile virus. J Clin Microbiol. 2005;43:5873‐5875. 10.1128/JCM.43.12.5873-5875.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fox JL, Hazel SL, Tobler LH, Busch MP. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol. 2006;13:33‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roque‐Afonso A‐M, Grangeot‐Keros L, Roquebert B, et al. Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J Clin Microbiol. 2004;42:5121‐5124. 10.1128/JCM.42.11.5121-5124.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gaudy‐Graffin C, Lesage G, Kousignian I, et al. Use of an anti‐hepatitis C virus (HCV) IgG avidity assay to identify recent HCV infection. J Clin Microbiol. 2010;48:3281‐3287. 10.1128/JCM.00303-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kanno A, Kazuyama Y. Immunoglobulin G antibody avidity assay for serodiagnosis of hepatitis C virus infection. J Med Virol. 2002;68:229‐233. [DOI] [PubMed] [Google Scholar]
- 74. Ward KN, Dhaliwal W, Ashworth KL, Clutterbuck EJ, Teo CG. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol. 1994;43:367‐372. [DOI] [PubMed] [Google Scholar]
- 75. De Souza VAUF, Fernandes S, Arau´jo ES, et al. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J Clin Microbiol. 2004;42:1782‐1784. 10.1128/JCM.42.4.1782-1784.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rauer S, Beitlich P, Neubert U, Rasiah U, Kaiser R. Avidity determination of Borrelia burgdorferi‐specific IgG antibodies in lyme disease. Scand J Infect Dis. 2009;33:809‐811. [DOI] [PubMed] [Google Scholar]
- 77. Lappalainen M, Hedman K. Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity. Ann 1st Supr Sanita. 2004;40:81‐88. [PubMed] [Google Scholar]
- 78. Chan KH, Sonnenberg K, Niedrig M, et al. Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection. Clin Vacc Immunol. 2007;14:1433‐1436. 10.1128/CVI.00056-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chan PKS, Lim P‐L, Liu EYM, Cheung JLK, Leung DTM, Sung JJY. Antibody avidity maturation during severe acute respiratory syndrome–associated coronavirus infection. JID. 2005;192:166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chan K‐H, Chan JF‐W, Tse H, et al. Cross‐reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect. 2013;67:130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Junker AK, Tilley P. Varicella‐zoster virus antibody avidity and subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119‐124. [DOI] [PubMed] [Google Scholar]
- 82. Martin KA, Junker AK, Thomas EE, Van Allen MI, Friedman JM. Occcurence of chickenpox during pregnancy in women seropositive for varicella‐zoster virus. J Infect Dis. 1994;170:991‐995. [DOI] [PubMed] [Google Scholar]
- 83. Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115‐1121. [DOI] [PubMed] [Google Scholar]
- 84. Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll‐like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nature Med. 2009;15:34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McCoy KD, Stoel M, Stettler R, et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362‐373. [DOI] [PubMed] [Google Scholar]
- 86. Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine‐induced protection. J Infect Dis. 2012;206:1542‐1548. [DOI] [PubMed] [Google Scholar]
- 87. Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. Correlation between Dengue‐specific neutralizing antibodies and serum avidity in primary and secondary Dengue virus 3 natural infections in humans. PLOS Neglect Trop Dis. 2013;7:e2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Edridge AWD, Kaczorowska J, Hoste ACR, et al. Human coronavirus reinfection dynamics: lessons for SARS‐CoV‐2. medRxiv 10.1101/2020.05.11.20086439 [DOI] [Google Scholar]
- 89. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS‐CoV‐2 infection and the potential for reinfection. J Gen Virol. 2020. 10.1099/jgv.0.001439 [DOI] [PMC free article] [PubMed] [Google Scholar]


