Abstract
Objectives
The purpose of this study was to assess coinfection rates of coronavirus disease 2019 (COVID‐19) with other respiratory infections on presentation.
Methods
This is a retrospective analysis of data from a 2 hospital academic medical centers and 2 urgent care centers during the initial 2 weeks of testing for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), March 10, 2020 to March 23, 2020. Testing was targeted toward high‐risk patients following US Centers for Disease Control and Prevention guidelines. Demographics include age group and sex. Laboratory test results included SARS‐CoV‐2, rapid influenza A/B, and upper respiratory pathogen nucleic acid detection. Patient demographics and coinfections are presented overall and by test results with descriptive statistics.
Results
Complete laboratory results from the first 2 weeks of testing were available for 471 emergency department patients and 117 urgent care center patients who were tested for SARS‐CoV. A total of 51 (8.7%) patients tested positive for COVID‐19 with only 1 of these patients also testing positive for another respiratory infection. One of the patients positive for COVID‐19 also tested positive for influenza A. Among the 537 patients who were screened and tested negative for COVID‐19, there were 33 (6.1%) patients who tested positive in the upper respiratory pathogen nucleic acid detection test.
Conclusion
In our study investigating coinfections among 51 patients testing positive for COVID‐19, 1 patient also tested positive for influenza A. Although we found limited coinfections in our emergency department and urgent care center patient populations, further research is needed to assess potential coinfection in patients with COVID‐19.
Keywords: coinfection, COVID‐19, emergency department, epidemic, pandemic, virus
1. INTRODUCTION
On March 10, 2020, the World Health Organization declared a global pandemic due to widespread infection with novel coronavirus COVID‐19 (coronavirus disease 2019) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Initial testing protocols from the US Centers for Disease Control and Prevention (CDC) for COVID‐19 for detection in patients with possible infection recommend that samples also should be first sent for influenza viruses along with respiratory panels for detection of parainfluenza virus, adenovirus, human rhinovirus, respiratory syncytial virus, Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae. 1
The problem with testing for coinfections in suspected COVID‐19 patients is that the presence of a positive upper respiratory pathogen nucleic acid detection (RPNA) test for viruses other than SARS‐CoV‐2 may suggest to the clinicians alternate explanations for the patients’ symptoms. The CDC continues to strongly encourage to test for other causes of respiratory illness. 1 Therefore, a positive RPNA test could potentially lead to failure to test for COVID‐19, thereby increasing exposure risks if a patient has a coinfection.
Initial investigations of patients with COVID‐19 in Wuhan, China, showed a low to non‐existent rate of coinfection with other respiratory viral pathogens. 2 , 3 However, a more recent study, in presumably cooler climates, have shown an increasing presence of coinfections (up to 50%), typically influenza or rhinovirus. 4
Because of the large variations in data, and the lack of experience with comprehensive testing for COVID‐19 along with other respiratory pathogens, we elected to look retrospectively at our own experience with testing. Such data could help in weighing the pros and cons of strategies or algorithms that involve respiratory pathogen panels or flu testing as a decision point for COVID‐19 testing.
2. METHODS
2.1. Study design and setting
This study is a retrospective analysis of data from an academic medical center with 2 hospitals and 2 urgent care centers in San Diego, California, during the initial 2 weeks of SARS‐CoV‐2 testing, March 10, 2020 to March 23, 2020. The hospitals have collective inpatient beds of 1000 with >85,000 emergency department (ED) visits each year, and the urgent care (UC) centers have about 28,000 visits each year, all located in suburban communities in the region. This study was approved by the institution's Human Subject Protections Program.
2.2. Study population
COVID‐19 testing was targeted toward high‐risk patients with the following known criteria as per the CDC guidelines that were consistent during the study period: patients presenting with symptoms concerning for COVID‐19 infection (fever and cough or shortness of breath), travel within 14 days to countries with high rates of infection (at that time China, Iran, Italy, Japan, and South Korea), or risk factors for infectious complications (including age or comorbid conditions), or the patient was a health care worker who could potentially expose others at risk. Patients with suspected COVID‐19 had nasal and throat‐swab specimens from the upper respiratory tract obtained and maintained in viral‐transport medium. Samples had SARS‐CoV‐2 confirmed by real‐time reverse transcription polymerase chain reaction (RT‐PCR; ePlex SARS‐CoV‐2 Test, GenMark Diagnostics, Carlsbad, CA).
The Bottom Line
COVID‐19 coinfection rates with other respiratory pathogens are currently unknown. In this study of 588 patients tested for COVID‐19, only 1 patient had a coinfection with influenza A.
On all patients suspected of COVID‐19, ED clinicians were encouraged to simultaneously collect an RPNA or influenza test. If that was positive, it would lessen the suspicion for COVID, although formal testing was pending. Sample pharyngeal swabs were often simultaneously tested for rapid influenza direct antigen testing (Abbott Diagnostics, Chicago, US) and/or an RPNA test via RT‐PCR (GenMark ePlex respiratory pathogens panel), which tests for the following viruses and other organisms: adenovirus, non‐SARS‐CoV‐2 coronavirus (229E, HKU1, NL63, OC43), metapneumovirus, rhinovirus/enterovirus, influenza A (H1N1, H1‐2009, H3), influenza B, parainfluenza, 1 , 2 , 3 , 4 respiratory syncytial virus (A,B), Chlamydia pneumoniae, and Mycoplasma pneumoniae.
2.3. Analysis
Data were queried from the shared electronic medical record (EPIC) using standard structured query language (SQL) queries. Data collected included demographics and laboratory test results. Demographics included age group (<18, 18–64, and 65 and older) and sex. Laboratory test results included those for SAR‐CoV‐2, rapid influenza A/B, and RPNA. Patient demographics and coinfections are presented overall and by test results with descriptive statistics.
3. RESULTS
A flow diagram of virology testing for patients during the first 2 weeks of COVID‐19 testing is presented in Figure 1. There were 972 patients tested for either influenza using the rapid test (65.8%), RPNA (61.4%), or COVID‐19 (60.5%). Among the 597 patients with RPNA viral testing, 353 (59.1%) also received COVID‐19 testing, whereas 500 (78.1%) patients with influenza testing also received COVID‐19 testing.
FIGURE 1.
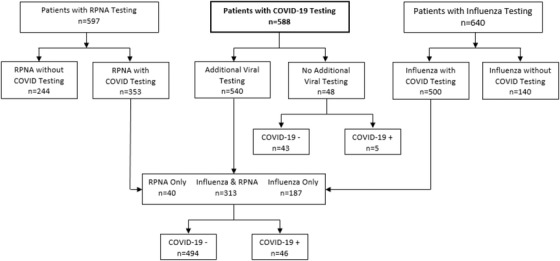
Flow diagram of virology testing for patients during the first 2 weeks of COVID‐19 testing. COVID, coronavirus; COVID‐19, coronavirus disease 2019; RPNA, upper respiratory pathogen nucleic acid detection
Among the 588 patients with SARS‐CoV‐2 test results, 471 were ED patients and 117 were seen in UC. The patients tested had a mean age of 47 (range 9–100), and an equal number of males and females were tested (n = 294 each). A total of 51 (8.7%) patients tested positive for SARS‐CoV‐2. Physicians followed protocols and restricted testing to only symptomatic patients with respiratory symptoms and/or fever. Of the 588 patients who received SARS‐CoV‐2 testing, 500 (85.0%) were also tested for influenza and 353 (60.0%) received a RPNA test, with 540 (91.8%) getting at least 1 of the 2 tests. Demographics and testing frequency are presented in Table 1.
TABLE 1.
Demographics and testing characteristics among patients who received a COVID‐19 test within the first 14 days of testing
| Demographics and testing | SARS‐CoV‐2 positive, N = 51; n (%) | SARS‐CoV‐2 negative, N = 537; n (%) | All SARS‐CoV‐2 tests, N = 588; n (%) |
|---|---|---|---|
| Age group | |||
| <18 | 0 (0.0) | 2 (0.3) | 2 (0.3) |
| 18–64 | 44 (86.3) | 438 (81.6) | 482 (82.0) |
| 65+ | 7 (13.7) | 97 (18.1) | 104 (17.7) |
| Sex | |||
| Male | 29 (56.9) | 265 (49.3) | 294 (50.0) |
| Female | 22 (43.1) | 272 (50.7) | 294 (50.0) |
| Influenza A/B test | 42 (82.4) | 458 (85.3) | 500 (85.0) |
| RPNA test | 27 (52.9) | 326 (60.7) | 353 (60.0) |
| Influenza A/B or RPNA test | 46 (90.2) | 494 (92.0) | 540 (91.8) |
| Testing | |||
| SARS‐CoV‐2 only | 5 (9.8) | 43 (8.0) | 48 (8.2) |
| SARS‐CoV‐2 and influenza | 19 (37.3) | 168(31.3) | 187 (31.8) |
| SARS‐CoV‐2 and RPNA | 4 (7.8) | 36 (6.7) | 40 (6.8) |
| SARS‐CoV‐2, influenza, and RPNA | 23 (45.1) | 290 (54.0) | 313 (53.2) |
COVID‐19, coronavirus disease 2019; RPNA, upper respiratory pathogen nucleic acid detection.
Only 1 of the patients positive for SARS‐CoV‐2 also simultaneously tested positive for any other coinfection. The case was an otherwise healthy 21‐year‐old male who returned to the United States from Italy 9 days earlier. He presented with 3 days of cough, congestion, and fever. He tested positive for both SARS‐CoV‐2 and influenza A by nucleic acid amplification.
Among the 537 patients who were screened and tested negative for SARS‐CoV‐2, there were 33 (6.1%) patients who tested positive in the RPNA test. A total of 4 (12.1%) of these patients tested positive for multiple organisms. The predominant respiratory pathogen detected was rhinovirus. There were also 2 influenza cases, both influenza A type. Influenza A/B and RPNA test results by SARS‐CoV‐2 status are reported in Table 2.
TABLE 2.
Influenza A/B and RPNA test results by COVID‐19 status
| Laboratory test | SARS‐CoV‐2 positive | SARS‐CoV‐2 negative | All SARS‐CoV‐2 tests |
|---|---|---|---|
| Influenza (rapid test) | 42 | 458 | 500 |
| Influenza A | 1 | 1 | 2 |
| Influenza B | 0 | 0 | 0 |
| RPNA | 27 | 326 | 353 |
|---|---|---|---|
| Adenovirus PCR | 0 | 0 | 0 |
| Coronavirus (non‐SARS‐CoV‐2) PCR | 0 | 4 | 4 |
| Chlamydophila pneumoniae PCR | 0 | 0 | 0 |
| Influenza A PCR | 0 | 1 | 1 |
| Influenza B PCR | 0 | 0 | 0 |
| Human metapneumovirus PCR | 0 | 10 | 10 |
| Mycoplasma pneumoniae PCR | 0 | 0 | 0 |
| Parainfluenza 1 PCR | 0 | 0 | 0 |
| Parainfluenza 2 PCR | 0 | 0 | 0 |
| Parainfluenza 3 PCR | 0 | 0 | 0 |
| Parainfluenza 4 PCR | 0 | 0 | 0 |
| Human rhinovirus/enterovirus PCR | 0 | 17 | 17 |
| Respiratory syncytial virus A PCR | 0 | 2 | 2 |
| Respiratory syncytial virus B PCR | 0 | 2 | 2 |
| Influenza A H1 PCR | 0 | 0 | 0 |
| Influenza A H1‐2009 PCR | 0 | 1 | 1 |
| Influenza A H3 PCR | 0 | 0 | 0 |
COVID‐19, coronavirus disease 2019; PCR, polymerase chain reaction; RPNA, upper respiratory pathogen nucleic acid detection.
4. LIMITATIONS
The main limitation of our study is the small sample size and lack of standardized testing, with the inclusion of only patients suspected of COVID‐19. The bias in sampling was restricting testing to symptomatic patients with COVID‐19 exposure or foreign travel. Therefore, we cannot comment on the incidence of COVID‐19 in a cohort of patients who ''ruled out'' by mere presence of another respiratory pathogen. The RPNA testing platform as well as sample collection practices could easily affect the accuracy of results. Of note, during this same period we had 2 patients positive for SARS‐CoV‐2 at 1 of our community hospitals who also tested positive for respiratory syncytial virus (RSV) in 1 case, and adenovirus and rhinovirus in the second, all on the film array respiratory panel (Biofire Diagnostics, Salt Lake City, UT). They tested negative on repeat with our GenMark ePlex respiratory pathogens panel. Also, a simple detection of a virus on a pharyngeal swab does not necessarily imply active infection with that virus.
5. DISCUSSION
COVID‐19 initially causes non‐specific symptoms with most being mild with cough, myalgia, and fever. 3 Therefore, it would make sense to not only suspect COVID‐19 if prevalent but also be suspicious for other respiratory illnesses such as influenza. Influenza was still active in the region with >500 cases reported a week during the first 2 weeks of March, with the majority being influenza A. A positive test for other respiratory pathogens on routine flu swab or RPNA could easily result in diagnosing a routine viral illness and potentially missing SARS‐CoV‐2, the cause of COVID‐19. Therefore, early testing strategies incorporating stepwise screening have the potential to miss many cases if COVID‐19 becomes an epidemic within any locality.
Coinfection with other respiratory viruses, before COVID‐19, has been described and should not be surprising. One study found that there was codetection of 2 or more viruses in 78% of patients requiring hospitalization for respiratory illness. 5 Of those, coinfection with human coronavirus (non‐SARS‐CoV‐2) together had an increased association with pediatric ICU admission. Adults also have been reported to have coinfections at a much lower rate, with 1 study showing only 2 of 232 outpatients with community‐acquired pneumonia testing positive for dual viral pathogens. 6 So in a non‐COVID‐19 series, respiratory coinfection with >1 viral pathogen occurs, but rarely in adults.
The low rate of coinfection, or codetection, of other respiratory pathogens in COVID‐positive patients is consistent with what was seen in Wuhan initially. 2 , 3 One study showed that none of the 99 initial SARS‐CoV‐2 positive patients had any other respiratory viruses detectable on pharyngeal swabs (RT‐PCR for influenza A, b, RSV, parainfluenza, adenovirus, severe acute respiratory syndrome [SARS], Middle East respiratory syndrome[MERS]). 2 Another series of 41 SARS‐CoV‐2‐positive patients had respiratory specimens tested for common viruses including RSV, adenovirus, parainfluenza, SARS, and MERS using real‐time RT‐PCR assays approved by the China Food and Drug Administration. 3 Although not explicitly stated, they reported no coinfections in their SARS‐CoV‐2‐positive patients.
More recent studies show that, in fact, coinfection is not entirely zero as initially thought. Researchers described how all COVID‐19‐positive patients from both Quingdao and Wuhan were simultaneously tested with indirect immunofluorescence for serum immunoglobulin M antibodies that detect the following 9 common respiratory pathogens: RSV, adenovirus, influenza A and B, parainfluenza, mycoplasma, chlamydia, legionella, and coxiella. 4 Of the 230 patients admitted in Qingdao, 24 had immunoglobulin M antibodies against other respiratory pathogens for a positive coinfection rate of 80% versus only 2.6% (1) in the 38 patients from Wuhan. Of note, the most common coinfections detected were influenza A (60%) and B (53%), followed by M pneumoniae and L pneumophila. This is starting to be seen in other areas of the world, including the simultaneous presence of influenza and SARS‐CoV‐2. 7
In published series so far, for adults, influenza is the most common coinfection seen in SARS‐CoV‐2‐positive patients, as was seen in our single case. A recent series of 116 SARS‐CoV‐2‐positive samples from Northern California showed an astounding rate of 7.5% (24) with 1 or more non‐SARS‐CoV‐2 pathogens. 8 The most common coinfections were rhinovirus/enterovirus, RSV, and non‐SARS‐Cov‐2 Coronaviridae. There are no details on testing platform, which in addition to local epidemiology could explain the stark difference in results. The reason some areas may have more coinfections may depend on the seasonal prevalence of other viruses because of weather or other epidemiological factors.
Most of the initial studies looking at coinfection were in adults. Pediatric studies show a higher rate of coinfection than seen in most adult series. In a series of 20 SARS‐CoV‐2‐positive patients, 8 (40%) had other pathogens detected on pharyngeal PCR swabs. 9 The predominant coinfections were influenza A and B (15%) and mycoplasma (20%) on pharyngeal respiratory viral swabs.
To conserve resources, the CDC, and many local health authorities including hospital systems, have recommended to test for flu and/or respiratory pathogens in patients being screened for suspected COVID‐19. 1 If those are negative, then one is to proceed with COVID‐19 testing. Unfortunately, that strategy would have missed the 1 COVID‐19 case in our series who tested positive for flu as well. Based on the increasing reports of higher coinfection rates and the advent of more expanded testing, one cannot recommend that as a general screening strategy. Although only 1 viral coinfection of 51 patients positive for SARS‐CoV‐2 was detected, the upper limit of the 95% confidence interval for 1/51 (1.96%) extends as high at 10.3%. Therefore, it is not surprising that that SARS‐CoV‐2 is being added to the next iteration of the diagnostic testing assay for respiratory pathogens, which will prove most useful as this pandemic unfolds.
Therefore, based on our experience and review of the literature in patients with respiratory illness who need testing for SARS‐CoV‐2 or another respiratory virus, one should consider testing for both SARS‐CoV‐2, regardless of preliminary testing, if done, and for flu or other respiratory pathogens. As this new virus continues to be a common cause of respiratory infection, any patient with cough, dyspnea, and/or fever should be suspect for COVID‐19 regardless of the preliminary testing results for other respiratory pathogens.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Edward M. Castillo, Christopher J. Coyne, and Christian A. Tomaszewski contributed to conception and design of the study. Edward M. Castillo and Jesse J. Brennan led development of the data analysis plan. Jesse J. Brennan conducted the data analysis. Edward M. Castillo and Christian A. Tomaszewski drafted the article, which all authors reviewed, revised, and approved.
Biography
Edward M. Castillo, PhD, MPH, is a Professor in the Department of Emergency Medicine at the University of California, San Diego.

Castillo EM, Coyne CJ, Brennan JJ, Tomaszewski CA. Rates of coinfection with other respiratory pathogens in patients positive for coronavirus disease 2019 (COVID‐19). JACEP Open 2020;1:592–596. 10.1002/emp2.12172
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Faheem W. Guirgis, MD.
REFERENCES
- 1. Center for Disease Control and Prevention . Criteria to guide evaluation and laboratory testing for COVID‐19. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 17, 2020.
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing Q, Li G, Xing Y, et al. Precautions are needed for COVID‐19 patients with coinfection of common respiratory pathogens. medRxiv. 2020. 10.1101/2020.02.29.20027698 [DOI] [Google Scholar]
- 5. Matsuno AK, Gagliardi TB, Paula FE, et al. Human coronavirus alone or in co‐infection with rhinovirus C is a risk factor for severe respiratory disease and admission to the pediatric intensive care unit: a one‐year study in Southeast Brazil. PloS One. 2019;14(6):e0217744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Li X, Wang W, et al. The prevalence of respiratory pathogens in adults with community‐acquired pneumonia in an outpatient cohort. Infect Drug Resist. 2019;12:2335‐2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan BE, Lim KGE, Chong VCL, Chan SSW, Ong KH, Kuperan P. COVID‐19 and mycoplasma pneumoniae coinfection. Am J Hematol. 2020;95(6):723‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. JAMA. 2020;323(20):2085‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]


