Editor
Patient concerns about the possible increased susceptibility to coronavirus disease 19 (COVID‐19) infection or a more severe course of the disease when treated with immunosuppressive therapy may lead to lower treatment compliance. 1 , 2
For this study, all psoriasis patients with a scheduled visit at two university dermatology departments in Prague, Czech Republic during the national lockdown (16 March to 24 April) due to the COVID‐19 were enrolled. At the time of the study, the number of confirmed COVID‐19 cases in Prague increased from 116 (8.4 cases per 100 000 inhabitants) to 1668 cases (122 cases per 100 000 inhabitants). Patients completed a standardized Hospital Anxiety and Depression Scale (HADS) questionnaire; only the anxiety subscale was used (HADS‐A). The presence of anxiety was defined as a HADS‐A score of ≥8. 3 The participants were asked to reply to the following statement: ‘I feel an increased risk of infection (complications) from coronavirus (COVID‐19) because of the type of treatment for my psoriasis’. Patients were advised to discontinue treatment only if they showed symptoms of COVID‐19 or were in high‐risk contact with a confirmed case of COVID‐19. Statistical analysis was performed using SPSS v24.0 (SPSS Inc., Chicago, IL).
In total, all 210 patients complied with the inclusion criteria and agreed to participate in the study: 117 (55.7%) patients on biologics, 47 (22.4%) on conventional immunosuppressive therapy and 46 (21.9%) on topical therapy. Demographic and clinical characteristics of the patients in the three study groups were similar (Table 1). None patient on biologics and only 4.3% (2/47) on conventional immunosuppressants discontinued therapy because of concerns about their treatment and COVID‐19 infection.
Table 1.
Demographic and clinical characteristics of the study participants (n = 210)
| Characteristics |
Biologics (n = 117) |
Conventional systemic (n = 47) |
Topical therapy (n = 46) |
|---|---|---|---|
| Gender | |||
| Male | 76 (65.0%) | 29 (61.7%) | 24 (52.2%) |
| Female | 41 (35.0%) | 18 (38.3%) | 22 (47.8%) |
| Age (years) | |||
| Average (range) | 47.2 (19–84) | 48.0 (22–82) | 47.0 (18–78) |
| Average PASI (range) | 1.3 (0–8) | 4.8 (0–30) | 4.3 (0–24) |
| Current therapy | |||
| Anti‐TNF‐α | 43 (36.8%) | ||
| Anti‐IL | 74 (63.2%) | ||
| Cyclosporine | 3 (6.4%) | ||
| Methotrexate (oral) | 44 (93.6%) |
PASI, Psoriasis Area and Severity Index.
The distribution of patients regarding an anxiety score (HADS‐A) of ≥8 was 24.8% for patients on biologics, 19.1% on conventional systemic and only 6.5% on topical therapy. The prevalence of anxiety in patients on biologics was similar to patients on conventional systemics (OR = 1.39, 95% CI, 0.60–3.22, P = 0.54) but significantly higher than in patients on topical therapy (OR = 4.72, 95% CI, 1.36–16.38, P < 0.01). The average anxiety score was slightly higher (P = 0.61) in the biologics group (OR = 5.34, 95% CI, 4.59–6.09) than in the conventional systemic group (OR = 4.98, 95% CI, 3.77–6.19) and significantly higher (P < 0.04) than in the topical therapy patients (OR = 3.98, 95% CI, 3.27–4.69).
Treatment safety concerns were significantly more common in the biologics‐treated patients, where 40.7% either agreed/strongly agreed of having experienced an increased risk of COVID‐19 infection as compared to 21.3% in the conventional systemic group (P < 0.01) and 10.9% in the topical therapy group (P < 0.00001) (Fig. 1).
Figure 1.
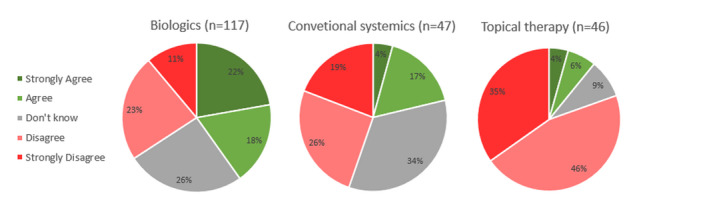
Patients' response to the statement, ‘I feel an increased risk of infection (complications) from coronavirus (COVID‐19) because of the type of treatment for my psoriasis’.
Psychological factors, such as depression or anxiety, have an important effect on treatment compliance. 4 Moreover, treatment safety is also a critical factor in a patient's decision to start or continue therapy. 5 Therefore, it can be expected that during the COVID‐19 pandemic, there may be a decrease in compliance, especially in psoriasis patients on immunomodulatory therapy. However, the results of our study show that although patients on biologic therapy had more anxiety and treatment safety concerns than patients in the other treatment groups, they did not want to discontinue or interrupt their treatment.
This study has limitations. Patient anxiety is affected by the severity of psoriasis, but in our study, patients on biologics had recently a milder case of the disease than patients in the other groups. It is also possible that the relationship between psoriasis treatment and anxiety could be indirect (e.g. due to unmeasured confounding) and not a direct consequence of the COVID‐19 pandemic.
In conclusion, the overall compliance of biologic treatment of patients with psoriasis during the COVID‐19 pandemic lockdown was extremely good despite expressing anxiety and more frequent concerns about the safety of their treatment compared to patients on other therapies.
Conflicts of interest
FR has received honoraria as a speaker and/or consultant for AbbVie, Celgene, Eli Lilly, Janssen, MSD, Novartis, Sanofi Genzyme, UCB. JH has received honoraria as a speaker and/or consultant for Novartis, LEO Pharma, Janssen, Eli Lilly, Sandoz, Celgene, Eucerin. ST has no conflict of interest. PB has received honoraria as a speaker and/or consultant for Eli Lilly, Janssen, Novartis, Leo Pharma. SG has received honoraria as a speaker and/or consultant for AbbVie, Celgene, Eli Lilly, Janssen, MSD, Novartis, Sanofi Genzyme, UCB, Leo Pharma. NV has received honoraria as a speaker for Novartis. MA reports fees for Advisory Boards from Abbvie, BMS. JH has received honoraria as a speaker and/or consultant for AbbVie, Celgene, Eli Lilly, Frankl Pharma, Janssen, Leo Pharma, Novartis, Novartis Global, Sanofi Aventis and Sanofi Genzyme.
Funding source
None.
References
- 1. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol 2020; 82: 1217–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conforti C, Giuffrida R, Dianzani C, Di Meo N, Zalaudek I. COVID‐19 and psoriasis: is it time to limit treatment with immunosuppressants? A call for action. Dermatol Ther 2020; e13298. https://di.org/10.1111/dth.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Julian LJ. Measures of anxiety: state‐trait anxiety inventory (STAI), Beck anxiety inventory (BAI), and Hospital anxiety and Depression scale‐anxiety (HADS‐A). Arthritis Care Res 2011; 63: S467–S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vangeli E, Bakhshi S, Baker A et al. A systematic review of factors associated with non‐adherence to treatment for immune‐mediated inflammatory diseases. Adv Ther 2015; 32: 983–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population‐based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol 2016; 17: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
[Correction added on 26 August 2020, after first online publication: the affiliations of authors have been corrected in this version.]


