Dear Editor:
Individuals with serious underlying medical conditions and those who are immunocompromised are at greatest risk for contracting coronavirus disease 2019 (COVID‐19). 1 This raises concern for patients with chronic inflammatory disorders, such as hidradenitis suppurativa (HS), who may seek virtual care to reduce COVID‐19 exposure. HS is a chronic, inflammatory skin disorder with risk factors, comorbidities, and complications which, when combined, may reduce an individual's defense against infection. However, HS alone does not appear to be a specific risk factor for COVID‐19. Nevertheless, managing HS virtually poses challenges because of complex treatment regimens involving lifestyle modifications and medical and surgical therapies. Here, we explore teledermatology management strategies and treatment considerations.
Optimizing teledermatology visits: To avoid emergency department/hospital visits and COVID‐19 exposure, teledermatology has replaced most in‐person consultations, posing unique challenges for HS management. For example, patients may feel reluctant and uncomfortable with exposing intertriginous (especially genital) areas via videoconferencing. Thus, we recommend patients send photos of private areas instead prior to their virtual appointment. To mitigate risks of data breach, photos can be sent via secure email or patient portal, which can then be directly uploaded onto their electronic medical record accessible to authorized individuals only. Furthermore, physical access to computers can be limited by locking workstations. For less sensitive body sites amenable to examination via videoconference, patients should be instructed to remove dressings in advance to minimize pain from rushed removals, and the same privacy standards used for in‐person visits should be maintained during videoconferencing (e.g., private consultation room). Since providers are unable to palpate fluctuant lesions that may require specific treatment, patients may be asked to apply pressure to lesions on camera. Finally, providers should consider providing management strategies and tips on when to arrange teledermatology to triage whether in‐person visits are required. Overall, even with optimized teledermatology, patients with HS may still require in‐person care (e.g., surgery), making HS different from other chronic inflammatory dermatoses (e.g., atopic dermatitis or psoriasis) that do not involve procedures and can be managed remotely.
Minimizing HS flares: Experts recommend educating patients on flare prevention strategies (diet alteration and trigger avoidance), having short‐term antibiotic prescriptions available, and having medications delivered to patients. At‐home management for HS flares includes warm compresses, benzoyl peroxide wash, topical anesthetics, resorcinol or dapsone 5% gel, and over‐the‐counter analgesics. Currently, deroofing, local anesthetic procedures, and intralesional injections may be offered, however, many HS surgeries have been postponed.
Treatment considerations for HS: Some biologics are associated with a slight increased risk of nasopharyngitis and upper respiratory tract infections (Fig. 1), 2 , 3 , 4 , 5 , 6 , 7 however, Frew et al. (2020) re‐analyzed the adalimumab trials and reported an incidence rate of serious infections of 2.14 per 100 patient years, which was not significantly different compared to placebo and comparable to other inflammatory conditions. 8 Overall, patients with well‐controlled HS, without signs/symptoms or a diagnosis of COVID‐19, can continue on biologics with strict adherence to protective measures (social distancing, hand hygiene, and avoiding sick contacts). Furthermore, emerging data suggest biologics may not be detrimental in the setting of COVID‐19 9 ; however, providers should use their best judgment based on the patient's risks. Renin–angiotensin–aldosterone system (RAAS) inhibitors can be continued in patients who are stable and at risk/evaluated for or diagnosed with COVID‐19, despite preclinical findings suggesting RAAS inhibitors increase angiotensin‐converting enzyme 2 (the receptor for severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]), as this may not translate to humans. 10 Moreover, abrupt withdrawal of RAAS inhibitors may cause clinical deterioration. 10
Figure 1.
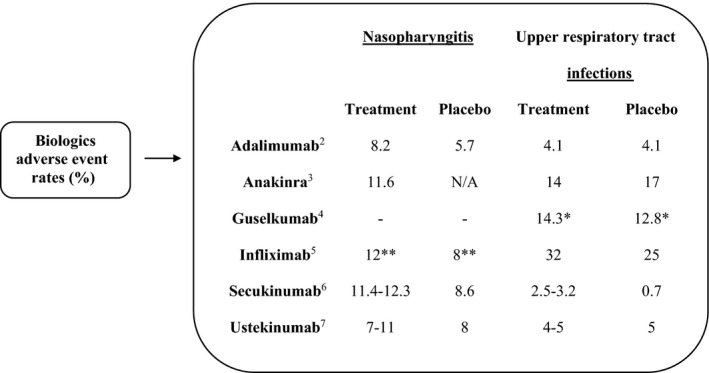
Adverse event rates of nasopharyngitis and upper respiratory tract infections in patients with inflammatory conditions on biologic therapies. Range of percentages reflects adverse event rates with increasing doses. Most biologics show no difference or a slight increase in rates of nasopharyngitis and upper respiratory tract infections compared to placebo. *Upper respiratory infection rates, including nasopharyngitis, upper respiratory tract infections, pharyngitis, and viral upper respiratory tract infections. **Pharyngitis rates. N/A, not available
In conclusion, patients with HS require special strategies in remote care. Providers should consider initiating virtual support groups for patients experiencing profound psychosocial suffering. 11 Furthermore, the decision to continue treatment should be determined on a case‐by‐case basis, taking into consideration patients' exposure risks for SARS‐CoV‐2, HS severity, risk of flaring, comorbidities, drug mechanism of action, and patients' response to retreatment. More data are needed to guide HS management in the context of COVID‐19, and we encourage practitioners to contribute to the following registries: (i) global hidradenitis suppurativa COVID‐19 Registry (https://hscovid.ucsf.edu) – identifies predictors of outcomes and informs HS management during COVID‐19; and (ii) COVID‐19 Dermatology Registry (https://www.aad.org/member/practice/coronavirus/registry) – identifies dermatologic manifestations of COVID‐19.
Conflicts of interest: Ms. Na‐Young C. Kang and Dr. Jennifer Hsiao have no conflicts of interest to disclose. Dr. Vivian Shi is a stock shareholder of Learn Health and has served as a consultant or investigator for or has received research funding from Sanofi/Regeneron, Eli Lilly, Dermira, Novartis, AbbVie, SUN Pharma, Pfizer, Leo, Menlo Therapeutics, Burt's Bees, GpSkin, and Skin Actives Scientific. Dr. Haley B. Naik has received grant support from AbbVie, consulting fees from 23 and Me and Johnson and Johnson, served on an advisory board for Boehringer Ingelheim, and is a board member of the Hidradenitis Suppurativa Foundation. Dr. Michelle A. Lowes has served on the advisory board for AbbVie, Janssen, and Viela Bio, and consulted for Almirall, BSN, Incyte, Janssen, Kymera, and XBiotech. Dr. Afsaneh Alavi received honoraria as a consultant, speaker, or advisory board participant from AbbVie, Actelion, Celgene, Galderma, GSK, Janssen, Leo Pharma, Novartis, Sanofi2Genzyme, and Bausch; received grants from AbbVie; and was a research investigator with AbbVie, Aristea, Asana, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Dermavant, Eli Lilly, Genetech, Glenmark, Incyte, Infla Rx, Janssen, Kyowa, Kymera, Leo Pharma, Merck Serono, Novartis, Pfizer, Regeneron, Roche, UCB, Xoma, and Xenon.
Funding source: None.
Original publication: The views expressed in this article are those of the authors, some of which have previously been presented in an online webinar and podcast. Alavi A, George R, Bunce P, Chavoshi S. Hidradenitis Suppurativa (HS) Heroes. Management of Hidradenitis Suppurativa in the Pandemic Era [Online Webinar]. 2020. Available at: https://bit.ly/2Kw9dxE (accessed May 11, 2020). Lev‐Tov H, Sivamani R, Hamzavi I, et al. Hidradenitis Suppurativa Foundation (HSF) News. Frequently Asked Questions about Hidradenitis Suppurativa (HS) and COVID‐19. 2020. Available at: https://www.hs-foundation.org/hidradenitis-suppurativa-treatment-and-covid-19-coronavirus/ (accessed May 11, 2020).
References
- 1. Centers for Disease Control and Prevention (CDC) . People who are at higher risk for severe illness. 2020. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐at‐higher‐risk.html (accessed 11 May 2020).
- 2. Blaszczak A, Trinidad JCL, Cartron AM. Adalimumab for treatment of hidradenitis suppurativa during the COVID‐19 pandemic: safety considerations. J Am Acad Dermatol. 22 April 2020. pii: S0190–9622(20)30606‐X. doi:10.1016/j.jaad.2020.04.030. [Epub ahead of print]; data from Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422–434. [DOI] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration (FDA) . Kineret® (anakinra) for injection, for subcutaneous use. 2001. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf (accessed 11 May 2020). [Google Scholar]
- 4. U.S. Food and Drug Administration (FDA) . Tremfya (guselkumab) injection, for subcutaneous use. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf (accessed 11 May 2020). [Google Scholar]
- 5. U.S. Food and Drug Administration (FDA) . Remicade (infliximab) lyophilized concentrate for injection, for intravenous use. 1998. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf (accessed 11 May 2020). [Google Scholar]
- 6. U.S. Food and Drug Administration (FDA) . CosentyxTM (secukinumab) injection, for subcutaneous use. 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125504s000lbl.pdf (accessed 11 May 2020). [Google Scholar]
- 7. U.S. Food and Drug Administration (FDA) . Stelara® (ustekinumab) injection, for subcutaneous or intravenous use. 2009. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf (accessed 11 May 2020). [Google Scholar]
- 8. Frew JW, Jiang CS, Singh N, et al. Malignancy and infection risk during adalimumab therapy in hidradenitis suppurativa. Clin Exp Dermatol 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020. [Epub ahead of print]2(6):e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaduganathan M, Vardeny O, Michel T, et al. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020; 382(17):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stout M. The role of virtual support groups for patients with hidradenitis suppurativa during the COVID‐19 pandemic. Int J Womens Dermatol 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


