Coagulopathy 1 and a prothrombotic diathesis with high D‐dimer and fibrinogen levels 2 are associated with coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Extensive thrombosis in small vessels and the microvasculature in lungs and extrapulmonary organs have been confirmed histologically. 3 Early studies showed that the venous thromboembolism (VTE) incidence in hospitalised patients with COVID‐19 can be as high as 25%, 4 and more recent studies have indicated this can be expanded to other macrovascular thrombotic complications, such as a higher than expected prevalence of pulmonary emboli in patients with COVID‐19. 5 , 6 The term ‘diffuse pulmonary intravascular coagulopathy’ has been proposed to describe the lung‐restricted vascular immunopathology associated with COVID‐19. This is distinct from disseminated intravascular coagulation in its early stages and is characterised by increased D‐dimer with normal platelet count and normal/elevated fibrinogen. 7 Increased VTE rate has been associated with higher D‐dimer level, and in the same study, an association between VTE and death was found. 8 Another study has shown that a D‐dimer level of >1 μg/ml was associated with a poorer prognosis. 9 Patients requiring mechanical ventilation who were treated with therapeutic anticoagulation had an in‐hospital mortality of 29·1% compared to 62·7% in patients who did not receive anticoagulation. Longer duration of anticoagulation was associated with a reduced risk of mortality. 10
Trials comparing prophylactic with treatment‐dose anticoagulation in inpatients with COVID‐19 have been proposed and indeed will be studied as part of the ‘Randomized Embedded Multifactorial Adaptive Platform for Community‐acquired Pneumonia’ (REMAP‐CAP) study. In this trial, the use of low‐molecular‐weight heparin (LMWH) or unfractionated heparin will be compared to standard pharmacological thromboprophylaxis. 11 However, it is still unknown if pre‐admission anticoagulation might provide a protective benefit in COVID‐19 infection. In theory, anticoagulants, or other antithrombotic agents such as antiplatelet drugs, might counteract the coagulopathic effects of COVID‐19, resulting in improved outcomes in these patients. Based on this hypothesis, we investigated the association between pre‐admission antiplatelet/anticoagulant use and COVID‐19 mortality.
The study population comprised those patients with confirmed COVID‐19 based on a polymerase chain reaction‐positive SARS‐CoV‐2 respiratory nasopharyngeal sample, while admitted as an inpatient in Brighton and Sussex University Hospitals NHS Trust between the 7 March and 9 April 2020. The case‐control group was constructed at a ratio of 1:2 cases to controls matching for age and sex, selecting from this overall population. A case was defined as being on an anticoagulant or antiplatelet agent before admission. Controls were then selected from the study population with a limited propensity matching by age and sex to two controls who were not taking the medication of interest using a ‘nearest neighbour’ method. Data on patients’ drug history were obtained using the Patient Administration System, which was also used to identify patient deaths up to 11 May 2020. This work was carried out as part of a service evaluation. Data collection and analysis were fully anonymised. Data were analysed using the Statistical Package for the Social Sciences (SPSS®; SPSS Inc., IBM Corp., Armonk, NY, USA) and plotted by Kaplan–Meier curves. Censored data represent end of follow‐up at the time of ceasing data collection.
Two populations were analysed. The first group compared those who did (cases) and did not (controls) take antiplatelet agents. The cases identified were 29, of which 62% (n = 18) were on aspirin, 28% (n = 8) on clopidogrel, and 10% (n = 3) on both. These were matched to controls (n = 58). The second group comprised 31 patients, who took anticoagulants, of which 23% (n = 7) were on warfarin, 39% (n = 12) apixaban, 3% (n = 1) dabigatran, 6% LMWH (n = 2), and 29% (n = 9) rivaroxaban. These were matched to controls (n = 62). The mean (SD) age for the ‘Anticoagulant’ and ‘Antiplatelet’ groups were 80 (9·2) and 79 (11·6) years respectively. Being on either an anticoagulant or antiplatelet agent before admission did not have a statistically significant effect on mortality in patients with COVID‐19 (P = 0·614 and P = 0·516 respectively, using the log‐rank test), suggesting no protective effect (Fig 1A,B). However, the evident confounder in this analysis is the comorbidity of cardiovascular disease, itself an established risk factor for increased mortality in COVID‐19, 12 and thrombotic disorders such as previous VTE or atrial fibrillation, which are common indications for the prescription of these classes of drugs. In our present data, we did not see any increased mortality amongst cases, suggesting that patients on antithrombotic agents have the same mortality rate as patients not taking these agents. Of note, within the Anticoagulant Group, warfarin use was seen to have an increased mortality risk upon comparison of survival distributions, although this was not statistically significant (P = 0·42) (Fig 2A). Similarly, within the Antiplatelet Group, aspirin use was seen to have a reduced mortality risk, but again this was not statistically significant (P = 0·62) (Fig 2B). Lastly, there was no difference in the percentage of patients admitted to the intensive care unit (ICU) between cases and controls in both the Anticoagulant and Antiplatelet groups (P = 0·47 and 0·83 respectively) (Table I).
Fig 1.
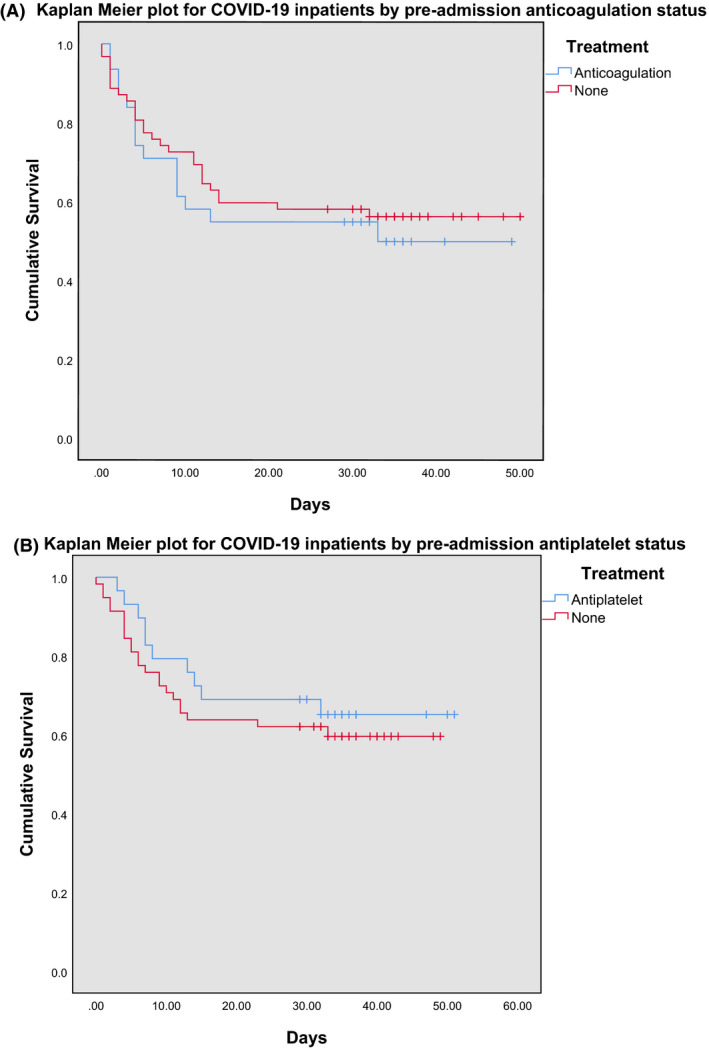
Kaplan–Meier plots of inpatients with COVID‐19 who were on (A) anticoagulation before admission versus those who were not and (B) antiplatelets before admission versus those who were not.
Fig 2.
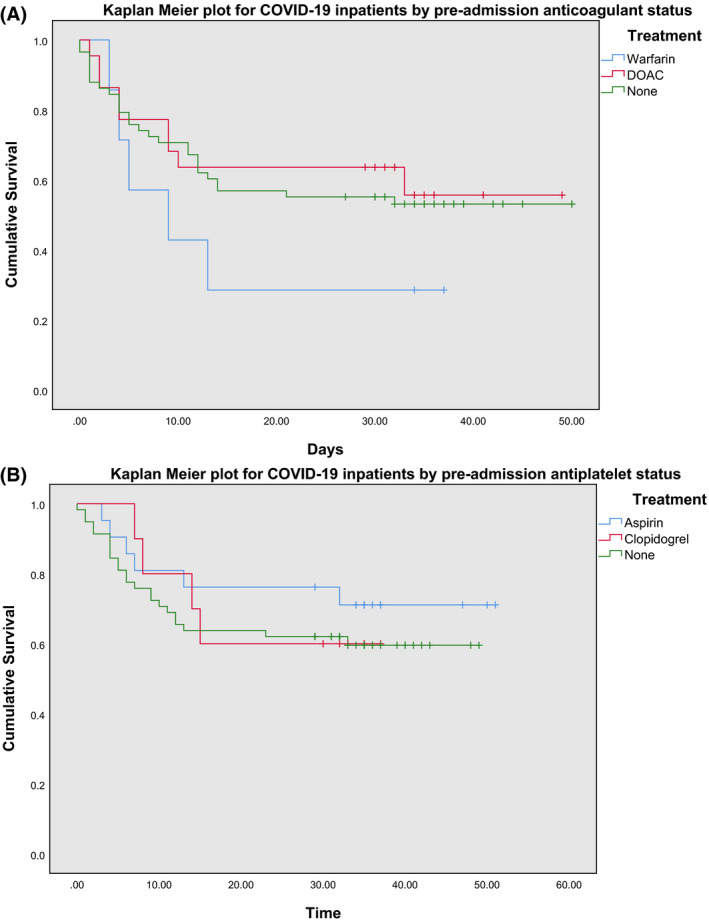
Kaplan–Meier plots of inpatients with COVID‐19 according to their (A) anticoagulant before admission and (B) antiplatelet before admission. DOAC, direct oral anticoagulants.
Table I.
Comparison of the patients admitted to the ICU between cases and controls in the Anticoagulant and Antiplatelet groups.
| Anticoagulant Group | Antiplatelet Group | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Number of patients | 31 | 62 | 29 | 58 |
| Age, years, mean | 80·5 | 80·2 | 79·3 | 79·3 |
| Patients admitted to ICU, % | 16·7 | 11·3 | 10·7 | 12·28 |
| P (chi squared) | 0·472 | 0·833 | ||
To conclude, our present study, although small, shows that patients taking antithrombotic therapy (anticoagulant or antiplatelet agents) at the time of infection with COVID‐19 do not have a significantly different mortality risk to those patients not taking these drugs. This could suggest these agents negate any potential increased mortality risk attributable to whichever disease the drugs had been prescribed for, but further data on comorbidities would be required to confirm this assertion. There were trends towards lower mortality in patients on aspirin, and towards increased mortality in patients on warfarin, but the limited sample size meant that these did not reach statistical significance. Trials are ongoing to assess the role of anticoagulation in the care of patients with COVID‐19 as inpatients. In addition, our present data suggest that larger scale investigation of pre‐admission anticoagulants or antiplatelet agents is needed, preferably adjusting for potential confounders such as cardiovascular disease, to establish the optimal protection of patients from the microangiopathic and thrombotic complications seen in COVID‐19.
Conflict of interest
No conflict of interest relevant to this letter was identified.
Author contributions
All authors contributed to the study design. Helena Sivaloganathan and Eleni E Ladikou performed data collection and analysis and drafted the manuscript. Timothy Chevassut reviewed the manuscript and supervised the project.
Acknowledgement
Dr Eleni Ladikou is an Academic Clinical Fellow (ACF) in Haematology, which is funded by National Institute for Health Research (NIHR). This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
H.S. and E.E.L. both contributed equally to this work and should both be considered first author.
References
- 1. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al‐Samkari H, Karp Leaf R, Dzik W, Carlson J, Fogerty A, Waheed A, et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS‐CoV2 infection. Blood. 2020. [Online ahead of print]. 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang T, Sun LX, Feng RE. [Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019] [Article in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:496–502. [DOI] [PubMed] [Google Scholar]
- 4. Songping C, Shuo C, Xiunan L, Shi L, Feng W. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok F, Kruip M, van der Meer N, Arbous M, Gommers D, Kant K, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;19:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stoneham SM, Milne KM, Nuttall E, Frew GH, Sturrock BR, Sivaloganathan H, et al. Thrombotic Risk in COVID‐19: a case series and case‐control study. Clin Med. 2020; [Online ahead of print]. 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGonagle D, O'Donnell J, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatology. 2020. [Online ahead of print]. 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Middeldorp S, Coppens M, van Haaps TF, Foppen, M. , Vlaar, A.P. & Müller, M.C. et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020. [Online ahead of print]. 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg B, Levin M, et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76:122–4. 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Angus DC, Berry S, Lewis RJ, Al‐Beidh F, Arabi Y, van Bentum‐Puijk W, et al. The randomized embedded multifactorial adaptive platform for community‐acquired pneumonia (REMAP‐CAP) study: rationale and design. Ann Am Thorac Soc. 2020. [Online ahead of print]. 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsushita K, Ding N, Kou M, Hu X, Chen M, Gao Y, et al. The relationship of COVID‐19 severity with cardiovascular disease and its traditional risk factors: A systematic review and meta‐analysis. medRxiv. 2020. [Online ahead of print]. 10.1101/2020.04.05.20054155. [DOI] [PMC free article] [PubMed] [Google Scholar]


