Abstract
The serious coronavirus disease‐2019 (COVID‐19) was first reported in December 2019 in Wuhan, China. COVID‐19 is an infectious disease caused by severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2). Angiotensin converting enzyme 2(ACE2) is the cellular receptor for SARS‐CoV‐2. Considering the critical roles of testicular cells for the transmission of genetic information between generations, we analyzed single‐cell RNA‐sequencing (scRNA‐seq) data of adult human testis. The mRNA expression of ACE2 was expressed in both germ cells and somatic cells. Moreover, the positive rate of ACE2 in testes of infertile men was higher than normal, which indicates that SARS‐CoV‐2 may cause reproductive disorders through pathway activated by ACE2 and the men with reproductive disorder may easily to be infected by SARS‐CoV‐2. The expression level of ACE2 was related to the age, and the mid‐aged with higher positive rate than young men testicular cells. Taken together, this research provides a biological background of the potential route for infection of SARS‐CoV‐2 and may enable rapid deciphering male‐related reproductive disorders induced by COVID‐19.
Keywords: ACE2, COVID‐19, reproductive disorder, scRNA‐seq, testicular cells
1. INTRODUCTION
A newly identified coronavirus, severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2), has been posing significant threats to public health. 1 The receptor of coronavirus determines the virus entry into the host cell and constitutes a target for the development of prophylactics and therapeutics. 2 , 3 SARS‐CoV‐2 with rapid global sp‐ read was that the SARS‐CoV‐2 binds ACE2 with higher affinity than SARS‐CoV. 4 Therefore, the analysis of ACE2 is essential to obtain the route of infection.
The testis is the key of the male reproductive system. Several studies have revealed the expression of ACE2 in testis; but the antibody detection might be subjected to non‐specificity issue. 5 The recently developed single‐cell RNA sequencing (scRNA‐seq) approaches can effectively delineate cell types and uncover heterogeneity that enables us to specifically detect ACE2 expression. 6 Infertility is the most serious type of reproductive disorder, here, we performed to analyse scRNA‐ seq data of testis from five donors with health and eight donors with infertility. 7 , 8
2. MATERIALS AND METHODS
2.1. Public dataset acquisition and processing
Single‐cell datasets (GSE106487, GSE112013) for human testis were obtained from the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/). GSE112013 consists of 6490 single cells and 3002 testicular single cells come from three normal young males. 7 GSE106487 includes samples from two adult normal males (N), seven obstructive azoospermia males (OA) and one nonobstructive azoospermia males (NOA). 8 Seurat (version 3.1.3) was used to read two datasets separately and discriminate different cell types. Firstly, datasets were normalized by NormalizeData method. Seurat FindClusters function was used to obtain cell cluster with resolution 0.7 in GSE106487 and 0.4 in GSE112013.
2.2. Identification of cell types and gene expression analysis
To obtain potential marker genes, Seurat function FindAllMarkers was used to per‐ form differential expression analysis with the default parameters. UMAP was used for visualization purposes. Besides, Canonical markers for cell clusters were selected and plotted using ggplot2 (version 3.2.1).
According to the previous research articles, SARS‐CoV‐2 tends to attack cells through receptor ACE2, therefore, we defined that ACE2 positive ratio is the number of ACE2 positive cells which expression value is larger than 0 within a cluster divided by a cluster total cell number. To clarify the relationship between ACE2 and regulatory protein, we used RNA and protein network data of ACE2 through GeneCards (https://www.genecards.org/). The relationship network data were imported into Cytoscape (version 3.7.2), and ACE2 was highlighted in yellow.
3. RESULTS
3.1. scRNA‐seq analysis of the human testicular cells
The scRNA‐seq offers new possibilities to address biological and medical questions, the diverse scRNA‐seq protocols and depths showed different efficient for transc‐ riptome quantification, so we performed to two data from different sources for analysis. 7 , 8 Group one with mid‐aged, the different donors were labelled for more accurate data analysis with cells coloured based on their donors origin (Figure S1A). 12 different cell types with four types of somatic cells and eight germ cell types were identified (Figures S1B and S2). We also analysed the data from young men for comparison, donors marked with a different colour (Figure S1C). After initial quality controls, 12 different cell types were identified in testis (Figures S1D and S3).
3.2. The ACE2 expression in human testicular cells
The mRNA of ACE2 were expressed in somatic and germ cells (Figure 1A,C). In mid‐aged, about 9% cells with ACE2 positive in SSCs, and Sertoli cells with higher ACE2 enrichment (90% of cells; Figure 1E). In young group, the mRNA expression of ACE2 was still identified in similar cell types (Figure 1B,C,F). Somatic cells are a key component of the testicular microenvironment, which play a significant role in the maintenance of SSCs and spermatogenesis (Figure 1A,B).
Figure 1.
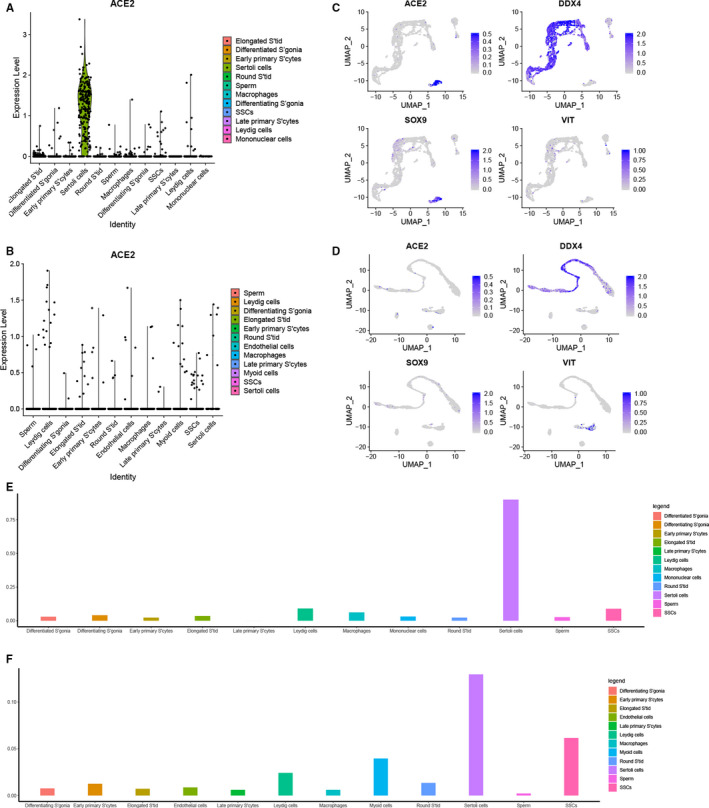
scRNA‐seq analysis of ACE2 in human testicular cells. A, Expression patterns (violin plot) showing the mRNA expression of ACE2 in the 12 clusters of the mid‐aged. The Sertoli cells are the highest positive cluster. B, The violin plot of mRNA expression of ACE2 across all cell types of the young group. C, UMAP plot showing the mRNA expression ACE2 across clusters of mid‐aged. The Sertoli cells (SOX9) are the highest ACE2‐positive cells, SSCs with the highest ACE2 positive rate in Germ cells (DDX4), and some Leydig cells (VIT) are positive. D, In young group, the ACE2‐positive cells shown in the Germ cells, Sertoli cells and Leydig cells. E, The percentage of ACE2‐positive cells in different type cells in mid‐aged, Sertoli cells with the highest positive cluster (over 90%). F, Sertoli cells are the highest cluster with ACE2‐positive cells in the young group
3.3. The analysis of ACE2 between healthy and infertility men
We noticed that the tendency was similar in two groups, but the specific data were different. The mid‐aged group included some pathological donors, so it was inferred that the expression of ACE2 is associated with reproductive disorders. To further de‐ termine the characteristic expression of ACE2, we analysed the proportion of ACE2 positive cells in different donors and found that the positive rate of the normal male was lower than that reproductive dysfunction (Figure 2A). The testicular cells of the infected SARS were seriously damaged, IgG was highly expressed in Sertoli cells and germ cells of the patients, and there was also expressed in the Leydig cells, which was similar to the distribution of ACE2 in the testis. 9
Figure 2.
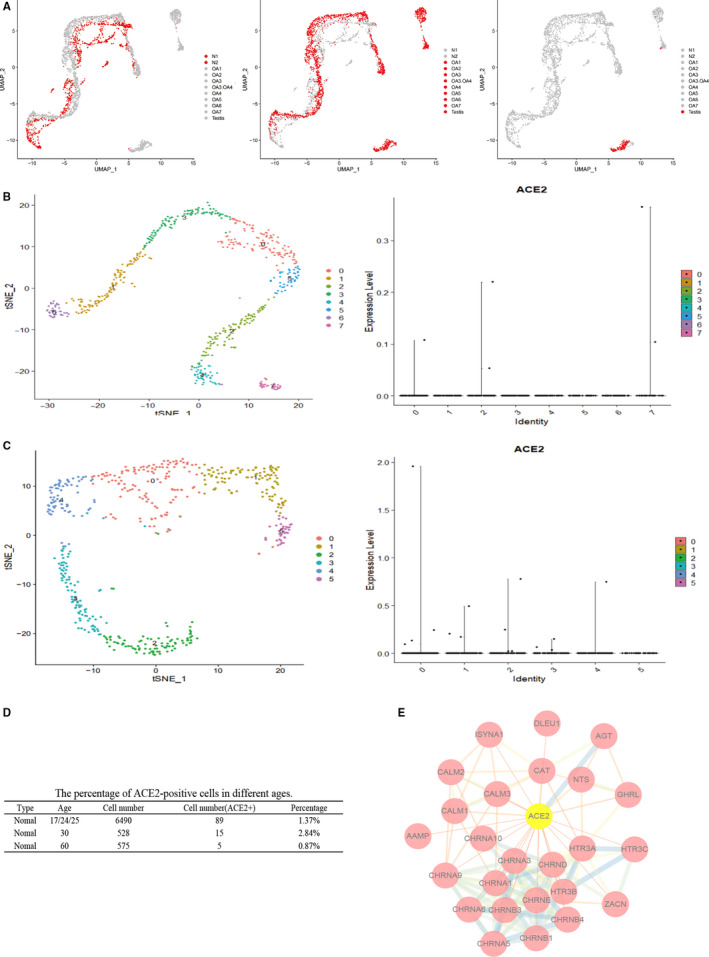
ACE2 expression in health and male infertility donors. A, UMAP plots showing the characteristics of N (left), OA (middle) and NOA (right) donors. The health are lower than that donors with reproductive dysfunction. B, tSNE plot of testicular cells to visualize cell‐type clusters (60 y old). The violin plot of ACE2 gene expression across all cell types in testis. C, tSNE plot of testicular cells to visualize cell‐type clusters (30 y old), and violin plot of ACE2 gene expression across all cell types in testis. D, The percentage of ACE2‐positive cells of different ages. In 30‐y‐old, men with the highest ACE2 positive rate, while about 20 y was relatively low, and the lowest ratio was the 60‐y‐old. E, ACE2 is interacting with proteins of inflammation‐related HTR3A and tumorigenesis‐related CHRNA1
3.4. The mRNA expression of ACE2 in testis at different ages
SARS‐CoV‐2 showed a various infection rate at different ages. 10 We analysed the positive cell rate of ACE2 in testis with healthy men, the highest was 2.84% in 30‐year‐old donor, followed by 20‐year‐old donors (1.37%), and the 60‐year‐old donors were about 0.87% (Figure 2B‐D). Moreover, the online data of GeneCards was analysed to explore potential regulatory mechanism of ACE2 in testis (Figure 2E). The results showed that ACE2 has related regulatory effects on 5‐Hydroxytryptamine Receptor 3A (HTR3A) and Cholinergic Receptor Nicotinic Alpha 1 (CHRNA1). HTR3A mediated apoptosis indirectly via TNF‐α production by enhancing serotonin release. 11 CHRNA1 interacts with inflammation‐related Leucocyte immunoglobulin‐like receptor subfamily B member 3 (L1LRB3). These data suggest the potential mechanism of abnormal function or even damage in patients infected with SARS‐CoV‐2.
4. DISCUSSION
ACE2 is a functional receptor for coronaviruses. 4 In this study, we explored scRNA‐seq data and found that the proportion of ACE2 positive cells in testis is more than 1%, which is higher than that in lung, indicated that the testis might serve as a high‐risk potential infection organ. 6 The testicular cell type with inflammation in SARS patients is consistent with the Sertoli cell cluster in our analysis, where ACE2 expression is abundant. 9 We noticed that the positive rate of ACE2 in testis of infertile was higher than normal men, which indicates that SARS‐CoV‐2 may cause reproductive disorders through abnormal activation of ACE2 pathway. One study found that SARS‐CoV‐2 in the semen of some male patients with COVID‐19, further implicating that testis is a portal of infection. 12 In addition, ACE2 is widely distributed in SSC, Sertoli cells, and Leydig cells. These findings indicate these cells as possible loci of infection and sexual transmission might be a part of the transmission routes.
SARS‐CoV‐2 had various infection rates in humans at different ages, and men had a higher prevalence than women. 13 Previous studies have shown that gender was an important factor affecting ACE2 concentrations in plasma. 13 Therefore, we investi‐ gated the positive rate of ACE2 in testicular cells of man at different ages. Here, we found that 30‐year‐old men with the highest ACE2 positive rate and 60‐year‐old men with the lowest ratio in testis. Men had higher mortality than women in the SARS epidemic. 14 Moreover, SARS‐CoV had a lower lethality rate for 2‐month‐old male mice than 8‐month‐old male mice and relatively higher mortality in middle‐aged mice. 15 These data suggest that viral infection might be associated with androgen secretion. Different treatment regimens should be appropriate for male and female patients considering androgen levels. Our studies may help with COVID‐19 prevention and control.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Qiaoyan Shen: Conceptualization (lead); data curation (equal); formal analysis (equal); methodology (equal); visualization (equal); writing‐original draft (lead); writing‐review & editing (lead). Xia Xiao: Data curation (equal); formal analysis (equal); methodology (equal); software (lead); writing‐original draft (equal); writing‐review & editing (equal). Aili Aierken: Writing‐original draft (supporting). Wei Yue: Writing‐review & editing (supporting). Xiaojie Wu: Writing‐review & editing (supporting). Mingzhi Liao: Conceptualization (equal); funding acquisition (lead); project administration (equal); writing‐original draft (equal); writing‐review & editing (equal). Jinlian Hua: Funding acquisition (lead); project administration (lead); writing‐original draft (equal); writing‐review & editing (equal).
Supporting information
Figure S1
Figure S2
Figure S3
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Program of China Stem Cell and Translational Research (JFYS 316 2016YFA0100203) and the Program of National Natural Science Foundation of China (31572399 and 61772431). Program of Shaanxi Province Science and Technology Innovation Team (2019TD‐036).
Shen Q, Xiao X, Aierken A, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS‐CoV‐2 infection. J Cell Mol Med. 2020;24:9472–9477. 10.1111/jcmm.15541
Shen and Xiao are equally contribute to this manuscript.
Contributor Information
Mingzhi Liao, Email: liaomz@nwsuaf.edu.cn.
Jinlian Hua, Email: jinlianhua@nwsuaf.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130(5):2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuan Y, Cao D, Zhang Y, et al. Cryo‐EM structures of MERS‐CoV and SARS‐ CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun. 2020;11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas GC, O’Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. [DOI] [PubMed] [Google Scholar]
- 6. Zou X, Chen KE, Zou J, et al. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo J, Grow EJ, Mlcochova H, et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;28(12):1141‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang M, Liu X, Chang G, et al. Single‐cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 2018;23(4):599‐614.e4. [DOI] [PubMed] [Google Scholar]
- 9. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syn‐drome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mikawa S, Kondo M, Kaji N, et al. Serotonin 3 receptor signaling regulates 5‐ fluorouracil‐mediated apoptosis indirectly via TNF‐alpha production by enhancing serotonin release from enterochromaffin cells. FASEB J. 2019;33(2):1669‐1680. [DOI] [PubMed] [Google Scholar]
- 12. Li D, Jin M, Bao P, et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J. 2020;41(19):1810‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Channappanavar R, Fett C, Mack M, et al. Sex‐based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


