Abstract
Objective
Coagulopathy is one of the characteristics observed in critically ill patients with coronavirus disease 2019 (COVID‐19). Antiphospholipid antibodies (aPLs) contribute to coagulopathy, though their role in COVID‐19 remains unclear. This study was undertaken to determine the prevalence and characteristics of aPLs in patients with COVID‐19.
Methods
Sera collected from 66 COVID‐19 patients who were critically ill and 13 COVID‐19 patients who were not critically ill were tested by chemiluminescence immunoassay for anticardiolipin antibodies (aCLs), anti–β2‐glycoprotein I (anti‐β2GPI) (IgG, IgM, and IgA), and IgG anti‐β2GPI–domain 1 (anti‐β2GPI–D1) and IgM and IgG anti–phosphatidylserine/prothrombin (anti‐PS/PT) antibodies were detected in the serum by enzyme‐linked immunosorbent assay.
Results
Of the 66 COVID‐19 patients in critical condition, aPLs were detected in 31 (47% ). Antiphospholipid antibodies were not present among COVID‐19 patients who were not in critical condition. The IgA anti‐β2GPI antibody was the most commonly observed aPL in patients with COVID‐19 and was present in 28.8% (19 of 66) of the critically ill patients, followed by IgA aCLs (17 of 66, or 25.8%) and IgG anti‐β2GPI (12 of 66, or 18.2%). For multiple aPLs, IgA anti‐β2GPI + IgA aCLs was the most common antibody profile observed (15 of 66, or 22.7%), followed by IgA anti‐β2GPI + IgA aCL + IgG anti‐β2GPI (10 of 66, or 15.2%). Antiphospholipid antibodies emerge ~35–39 days after disease onset. A dynamic analysis of aPLs revealed 4 patterns based on the persistence or transient appearance of the aPLs. Patients with multiple aPLs had a significantly higher incidence of cerebral infarction compared to patients who were negative for aPLs (P = 0.023).
Conclusion
Antiphospholipid antibodies were common in critically ill patients with COVID‐19. Repeated testing demonstrating medium to high titers of aPLs and the number of aPL types a patient is positive for may help in identifying patients who are at risk of developing cerebral infarction. Antiphospholipid antibodies may be transient and disappear within a few weeks, but in genetically predisposed patients, COVID‐19 may trigger the development of an autoimmune condition similar to the antiphospholipid syndrome (APS), referred to as “COVID‐19–induced APS‐like syndrome.” Long‐term follow‐up of COVID‐19 patients who are positive for aPLs would be of great importance in understanding the pathogenesis of this novel coronavirus.
INTRODUCTION
In patients affected with coronavirus disease 2019 (COVID‐19) who are critically ill, we and other investigators have observed that the disease is associated with a proinflammatory and hypercoagulable state and an increased risk of thrombotic events (i.e., pulmonary embolism and cerebral infarction), which are characterized by marked elevations in the levels of d‐dimers (1, 2, 3, 4, 5, 6). Currently, the etiology leading to hypercoagulability in COVID‐19 remains unclear.
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by the presence of antiphospholipid antibodies (aPLs) and a wide series of clinical manifestations, from recurrent arterial and/or venous thrombotic events to recurrent fetal loss. Antiphospholipid antibodies have long been considered as one of the contributors to a hypercoagulable state and to the development of the following thrombotic events. In addition to the pathogenic role in APS, aPLs are crucial to the diagnosis of APS. The 2006 criteria for APS recommend that routine tests for the presence of lupus anticoagulant (LAC), IgM and/or IgG anticardiolipin (aCL), and IgM and/or IgG anti–β2‐glycoprotein I (anti‐β2GPI) antibodies be conducted (7). In addition, the 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report highlighted non‐criteria aPLs, including IgA anti‐β2GPI, IgM/IgG anti‐phosphatidylserine/prothrombin (anti‐PS/PT), and anti‐β2GPI–domain I (anti‐β2GPI–DI) antibodies, as being associated with APS, especially seronegative APS (SNAPS) (8).
We have previously reported the presence of aPLs in 3 critically ill patients with COVID‐19 (9). However, it remains unclear whether these aPLs are pathogenic or whether they are persistent. In this study, we summarize the prevalence and characteristics of aPLs in 66 critically ill patients with COVID‐19 and identify clinical features based on the presence of aPLs.
PATIENTS AND METHODS
Clinical settings and patients
Consecutive critically ill patients with suspected COVID‐19 who were admitted to an intensive care unit (ICU) designated for patients with COVID‐19 were included in this cross‐sectional study. This unit, which was managed by a multidisciplinary team from Peking Union Medical College Hospital (PUMCH) in the Sino‐French New City Branch of Tongji Hospital (Wuhan, China), was set up on an emergency basis in order to treat the most critically ill patients during the outbreak of COVID‐19. The criterion for inclusion was any patient identified as being treated in our ICU. The criterion for exclusion were as follows: 1) any patient who was not diagnosed as having COVID‐19 and 2) any patient with COVID‐19 who was not assessed for aPLs. A total of 66 COVID‐19 patients in critical condition were included in the final results of this study.
COVID‐19 patients who visited the fever clinic at PUMCH in Beijing, China were also included in the present study. The criterion for inclusion was any consecutive patient who visited the fever clinic at PUMCH in Beijing. The criterion for exclusion was any patient who was not diagnosed as having COVID‐19. A total of 13 patients with COVID‐19 from the Beijing clinic were included in the present study, none of whom were critically ill. Diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2) infection was confirmed in all patients by reverse transcription–polymerase chain reaction (RT‐PCR) or serologic testing according to the Chinese Recommendations for Diagnosis and Treatment of Novel Coronavirus Infection (Pilot 7th version). Clinical characteristics and laboratories parameters at the time of admission, which were collected from electronic medical records, are listed in Table 1. The study was approved by the Research Ethics Commission of PUMCH, and the requirement for informed consent was waived by the Ethics Commission (ZS‐2303).
Table 1.
Demographic, clinical characteristics, and laboratory findings of patients infected with COVID‐19*
| Characteristics | Patients who were critically ill (n = 66) | |||
|---|---|---|---|---|
|
Patients positive for aPLs (n = 31) |
||||
|
Negative for aPLs (n = 35) |
Single/multiplelow (n = 16) |
Multiplemedium/high (n = 15) |
Patients who were not critically ill (n = 13) |
|
| Demographic characteristics | ||||
| Age, mean ± SD years | 64.5 ± 12.3 | 66.5 ± 13.3 | 65.2 ± 7.5 | 35.2 ± 19.3 |
| Sex, female/male | 17/18 | 5/11 | 5/10 | 7/6 |
| Comorbidity | ||||
| Hypertension | 17 (48.6) | 8 (50.0) | 8 (53.3) | 0 |
| Diabetes | 6 (17.1) | 3 (18.8) | 4 (26.7) | 0 |
| Coronary heart disease | 8 (22.9) | 0 | 2 (13.3) | 0 |
| Lung disease | 5 (14.3) | 1 (6.2) | 1 (6.7) | 0 |
| Carcinoma | 1 (2.9) | 1 (6.2) | 2 (13.3) | 0 |
| Chronic kidney disease | 0 | 0 | 1 (6.7) | 0 |
| Chronic liver disease | 4 (11.4) | 0 | 1 (6.7) | 1 (9.1) |
| Autoimmune diseases | 2 (5.7) | 0 | 0 | 0 |
| Thrombotic history | ||||
| Cerebral infarction | 4 (11.4) | 3 (18.8) | 2 (13.3) | 0 |
| Myocardial infarction | 1 (2.9) | 1 (6.2) | 0 | 0 |
| Other thrombotic events | 0 | 0 | 0 | 0 |
| Symptoms on admission | ||||
| Fever (temperature ≥37.3°C) | 31 (88.6) | 14 (87.5) | 13 (86.7) | 8 (61.5) |
| Cough | 32 (91.4) | 12 (75.0) | 10 (66.7) | 9 (69.2) |
| Sputum | 12 (34.3) | 6 (37.5) | 4 (26.7) | 0 |
| Dyspnea | 28 (80.0) | 15 (93.8) | 11 (73.3) | 0 |
| Myalgia | 9 (25.7) | 4 (25.0) | 3 (20.0) | 0 |
| Fatigue | 15 (42.9) | 3 (18.8) | 8 (53.3) | 0 |
| Diarrhea | 12 (34.3) | 3 (18.8) | 2 (13.3) | 0 |
| Headache | 6 (17.1) | 2 (12.5) | 3 (20.0) | 0 |
| Nausea or vomiting | 9 (25.7) | 1 (6.2) | 3 (20.0) | 0 |
| Disease severity status | ||||
| General | 0 | 0 | 0 | 12 (92.3) |
| Severe | 0 | 0 | 0 | 1 (7.7) |
| Critical | 35 (100) | 16 (100) | 15 (100) | 0 |
| ARDS | 12 (34.3) | 7 (43.8) | 6 (40.0) | 0 |
| Respiratory failure | 23 (65.7) | 13 (81.2) | 11 (73.3) | 0 |
| Laboratory findings on admission, mean ± SD | ||||
| White blood cell count, 109/liter | 13.5 ± 6.3 | 14.3 ± 7.1 | 13.5 ± 7.2 | 7.3 ± 2.4 |
| Total neutrophil count, 109/liter | 12.1 ± 5.9 | 12.1 ± 6.8 | 12.1 ± 6.7 | 3.3 ± 1.6 |
| Total lymphocyte count, 109/liter | 0.6 ± 0.6 | 0.8 ± 0.6 | 0.7 ± 0.4 | 1.7 ± 0.7 |
| Red blood cell count, 1012/liter | 3.5 ± 0.9 | 4.1 ± 1.2 | 3.5 ± 0.6 | 4.5 ± 0.6 |
| Platelets, 109/liter | 150.6 ± 102.9 | 177.9 ± 83.9 | 185.0 ± 83.2 | 223.0 ± 62.8 |
| Hemoglobin, gm/liter | 108.5 ± 23.7 | 120.8 ± 28.3 | 107.3 ± 22.2 | 134.1 ± 14.8 |
| ALT, units/liter | 38.1 ± 64.2 | 68.4 ± 165.7 | 28.9 ± 19.5 | 14.2 ± 7.5 |
| AST, units/liter | 37.8 ± 30.7 | 180.4 ± 583.7 | 34.0 ± 18.7 | – |
| LDH, units/liter | 510.1 ± 292.9 | 533.6 ± 458.8 | 447.9 ± 218.5 | – |
| Creatinine level, µmoles/liter | 106.6 ±125.9 | 74.6 ± 40.5 | 76.3 ± 37.2 | 52.3 ± 23.5 |
| EGFR, ml/minute/1.7 3m2 | 83.0 ± 35.0 | 89.8 ± 31.7 | 86.9 ± 24.8 | – |
| High‐sensitivity cardiac troponin I, pg/ml | 594.9 ± 2,410.0 | 607.0 ± 1,921.2 | 215.7 ± 497.6 | – |
| NT‐proBNP, pg/ml | 3,029.6 ± 5,306.6 | 1,756.2 ± 2,189.2 | 2,016.9 ± 2,217.6 | – |
| Prothrombin time, seconds | 17.6 ± 3.5 | 17.6 ± 7.5 | 16.1 ± 1.0 | – |
| APTT, seconds | 45.4 ± 21.0 | 45.8 ± 7.6 | 41.36 ± 6.44 | – |
| Fibrinogen, gn/liter | 3.6 ± 2.1 | 4.8 ± 1.6 | 4.5 ± 1.2 | – |
| d‐dimer, μg/liter | 10.9 ± 8.8 | 10.2 ± 9.0 | 8.9 ± 7.6 | – |
| Procalcitonin, ng/ml | 0.8 ± 1.9 | 0.3 ± 0.4 | 1.2 ± 2.0 | 0.2 ± 0.1 |
| High‐sensitivity CRP, mg/liter | 88.7 ± 84.3 | 98.1 ± 57.6 | 99.5 ± 51.8 | – |
| Interleukin‐6, pg/ml | 289.5 ± 877.5 | 277.3 ± 539.1 | 103.1 ± 125.3 | – |
| Treatments | ||||
| Corticosteroids | 27 (77.1) | 12 (75.0) | 10 (66.7) | NA |
| Intravenous immunoglobulin | 18 (51.4) | 8 (50.0) | 11 (73.3) | NA |
| Noninvasive mechanical ventilation | 17 (48.6) | 11 (68.8) | 7 (46.7) | NA |
| Invasive mechanical ventilation | 28 (80.0) | 14 (87.5) | 15 (100) | NA |
| Anticoagulant therapy | 19 (54.3) | 12 (75.0) | 9 (60.0) | NA |
| ECMO | 3 (8.6) | 1 (6.2) | 3 (15.0) | NA |
| Thrombotic events during COVID‐19 infection | ||||
| Arterial thrombosis | – | – | – | – |
| Cerebral infarction | 0 | 0 | 5 (33.3) | 0 |
| Myocardial infarction | 0 | 0 | 1 (6.7) | 0 |
| Venous thrombosis | ||||
| Large vein | 0 | 0 | 2 (13.3) | 0 |
| Distal vein | 10 (28.6) | 3 (18.6) | 4 (26.7) | 0 |
Except where indicated, values are the number (%). Patients positive for a single antiphospholipid antibody (aPL) or positive for more than 1 aPL with titers of all aPLs as ≤40 chemiluminescent units were classified as single/multiplelow patients. Patients positive for more than 1 aPL with titers (for at least 1 of the aPLs) of >40 chemiluminescent units were classified as multiple medium/high patients. When assessing the incidence of cerebral infarction during coronavirus disease 2019 (COVID‐19) infection, the occurrence of cerebral infarction differed between the patient groups, with some of the differences being significant, as follows: patients who were critically ill versus patients who were not critically ill, P = 0.010; patients who were positive for multiple aPLs versus patients who were negative for all aPLs, P = 0.023; patients who were positive for multiple aPLs versus patients who were positive for a single aPL, P = 0.101. P values were calculated with a Kruskal‐Wallis test followed by Dunnett’s T2 test. ALT = alanine aminotransaminase; APTT = activated partial thromboplastin time; ARDS = acute respiratory distress syndrome; AST = aspartate aminotransaminase; CHD = coronary heart disease; EGFR = estimated glomerular filtration rate; HFNC = high‐flow nasal cannula; CRP = C‐reactive protein; ECMO = extracorporeal membrane oxygenation; IMV = invasive mechanical ventilation; IVIG = intravenous immunoglobulin; LDH = lactate dehydrogenase; NIMV = noninvasive mechanical ventilation; NA = not applicable; NT‐proBNP = N‐terminal pro–brain type natriuretic peptide.
Detection of aPLs in serum samples
Serum aCL and anti‐β2GPI (IgG, IgM, and IgA) and IgG anti‐β2GPI–DI were determined by the chemiluminescence immunoassay (Inova) (10), with cutoff values for positivity set at >20 chemiluminescent units according to the manufacturer’s recommendations. IgG/IgM anti‐PS/PT were determined by enzyme‐linked immunosorbent assay (Inova) (11), and cutoff values for positivity were set at >30 chemiluminescent units according to the manufacturer’s recommendations.
Lupus anticoagulant
Detection of LAC in human citrated plasma was performed by HemosIL dilute Russell’s viper venom time (dRVVT) screening and HemosIL dRVVT confirmation assays, as recommended by the International Society on Thrombosis and Haemostasis.
Statistical analysis
Where appropriate, Mann‐Whitney U test, chi‐square test, or Fisher’s exact test were used to compare differences between patients who were positive for aPLs and those who were negative for aPLs. The Kruskal‐Wallis test followed by Dunnett’s multiple comparison test was used to compare differences between patients who were negative for all aPLs, patients who were positive for a single aPL or a low number of multiple aPLs, and patients who had medium to high titers of multiple aPLs. P values less than 0.05 (2‐sided) were considered statistically significant. All statistical analyses were performed using SPSS software version 20 (SPSS Inc).
RESULTS
We first determined the prevalence and characteristics of aPLs in patients with COVID‐19. Using the manufacturer’s recommended cutoff value of >20 chemiluminescent units, aPLs were detected in 47% of patients in critical condition (31 of 66) but not in patients who were not critically ill (Table 2). A previous study has shown that moderate to high titers of aPLs are more clinically relevant in identifying patients who are at risk of developing thrombosis in APS (12). As such, we re‐analyzed the prevalence of aPLs using the cutoff value of >40 chemiluminescent units. Antiphospholipid antibodies were present in 31.8% of critically ill patients (21 of 66).
Table 2.
Prevalence and characteristics of aPLs in patients with COVID‐19*
| Single or multiple aPLs |
Critically ill patients (n = 66) |
Patients who were not critically ill (n = 13) |
|---|---|---|
| Any aPL | 31 (47.0) | 0 |
| Single aPL | ||
| aCL | ||
| IgA | 17 (25.8) | 0 |
| IgG | 4 (6.0) | 0 |
| IgM | 2 (3.0) | 0 |
| LAC | 2 (3.0) | 0 |
| Anti‐β2GPI | ||
| IgA | 19 (28.8) | 0 |
| IgG | 12 (18.2) | 0 |
| IgM | 1 (1.5) | 0 |
| IgG–D1 | 2 (3.0) | 0 |
| Anti‐PS/PT | ||
| IgM | 7 (10.6) | 0 |
| IgG | 0 | 0 |
| Multiple aPLs | ||
| IgA aCL + IgA anti‐β2GPI | 15 (22.7) | 0 |
| IgM aCL + IgM anti‐β2GPI | 1 (1.5) | 0 |
| IgA anti‐β2GPI + IgG anti‐β2GPI | 1 (1.5) | 0 |
| LAC + IgA aCL + IgA anti‐β2GPI | 1 (1.5) | 0 |
| IgA aCL + IgA anti‐β2GPI + IgG anti‐β2GPI | 10 (15.2) | 0 |
| IgA aCL + IgG anti‐β2GPI + IgM aCL | 1 (1.5) | 0 |
| IgA aCL + IgA anti‐β2GPI + IgM anti–PS/PT | 1 (1.5) | 0 |
| IgA aCL + IgG aCL + IgA anti‐β2GPI + IgG anti‐β2GPI | 4 (6.1) | 0 |
| LAC + IgA aCL + IgG aCL + IgA anti‐β2GPI + IgG anti‐β2GPI | 1 (1.5) | 0 |
Values are the number (%). Cutoff values for positivity for all antiphospholipid antibodies (aPLs) except IgM/IgG anti‐phosphatidylserine/prothrombin (anti‐PS/PT) antibodies were set at >20 chemiluminescent units based on the recommendations of the manufacturer. Cutoff values for positivity for IgM/IgG anti‐PS/PT antibodies were set at >30 chemiluminescent units according to the manufacturer’s recommendations. COVID‐19 = coronavirus disease 2019; aCLs = anticardiolipin antibodies; LAC = lupus anticoagulant; anti‐β2GPI–D1 = anti‐β2–glycoprotein domain 1.
Next, the prevalence of each aPL was assessed. For single aPLs, IgA anti‐β2GPI was the most common type of aPL observed, present in 19 (28.8%) of 66 critically ill patients and 19 (61.3%) of 31 patients positive for aPLs, followed by IgA aCL (in 17 [25.8%] of 66 critically ill patients and 17 [54.8%] of 31 patients positive for aPLs), and IgG anti‐β2GPI (present in 12 [18.2%] of 66 critically ill patients and 12 [38.7%] of 31 patients positive for aPLs). For multiple aPLs, IgA anti‐β2GPI + IgA aCL was the most common antibody profile observed (in 15 [22.7%] of 66 critically ill patients), followed by IgA anti‐β2GPI + IgA aCL + IgG anti‐β2GPI (in 10 [15.2%] of 66 critically ill patients]) and IgA aCL + IgG aCL + IgA anti‐β2GPI + IgG anti‐β2GPI (present in 4 [6.1%] of 66 critically ill patients). All 66 critically ill patients were screened for the presence of LAC, with 2 patients testing positive for LAC. These findings suggest that COVID‐19 preferentially induced aPLs of the IgA isotype and, to a lesser extent, aPLs of the IgG isotype.
Next, we determined when aPLs emerged in those patients who were positive for these antibodies. Among the 31 patients positive for aPLs, serum was obtained from 10 patients who showed aPL negativity at an early time point after disease onset and aPL positivity at a later time point. Analysis of these patients’ sera revealed that aPLs emerged a median of ~39 days after disease onset (Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41425/abstract). Taken together, these findings demonstrate that aPLs emerge later in the disease course, suggesting that critically ill patients who have a longer disease duration are likely to have aPLs.
Dynamic changes in the both the numbers and titers of aPLs during the course of COVID‐19 in critically ill patients were investigated further. Due to the retrospective nature of this analysis, data on from multiple time points during which serum was tested for aPLs were only available for 6 patients (Figure 1). Generally, types and titers of aPLs increased from a single type of aPL with low titers to multiple types of aPLs with high titers. For the later time points, those 6 patients exhibited different antibody patterns. In patient 1, medium levels of IgG anti‐β2GPI were maintained despite interventions with plasma exchanges (Figure 1A). In patients 2 and 3, medium levels of IgA anti‐β2GPI + IgA aCLs were maintained after a transient appearance of IgG anti‐β2GPI (Figure 1B). In patients 4 and 5, aPLs were transient and disappeared at later time points (Figure 1C). In patient 6, high levels of IgA aCL+ IgA anti‐β2GPI + IgG anti‐β2GPI were maintained for ~2 weeks (Figure 1D). These results suggest that levels of aPLs fluctuate and exhibit different dynamic patterns among different patients with COVID‐19.
Figure 1.
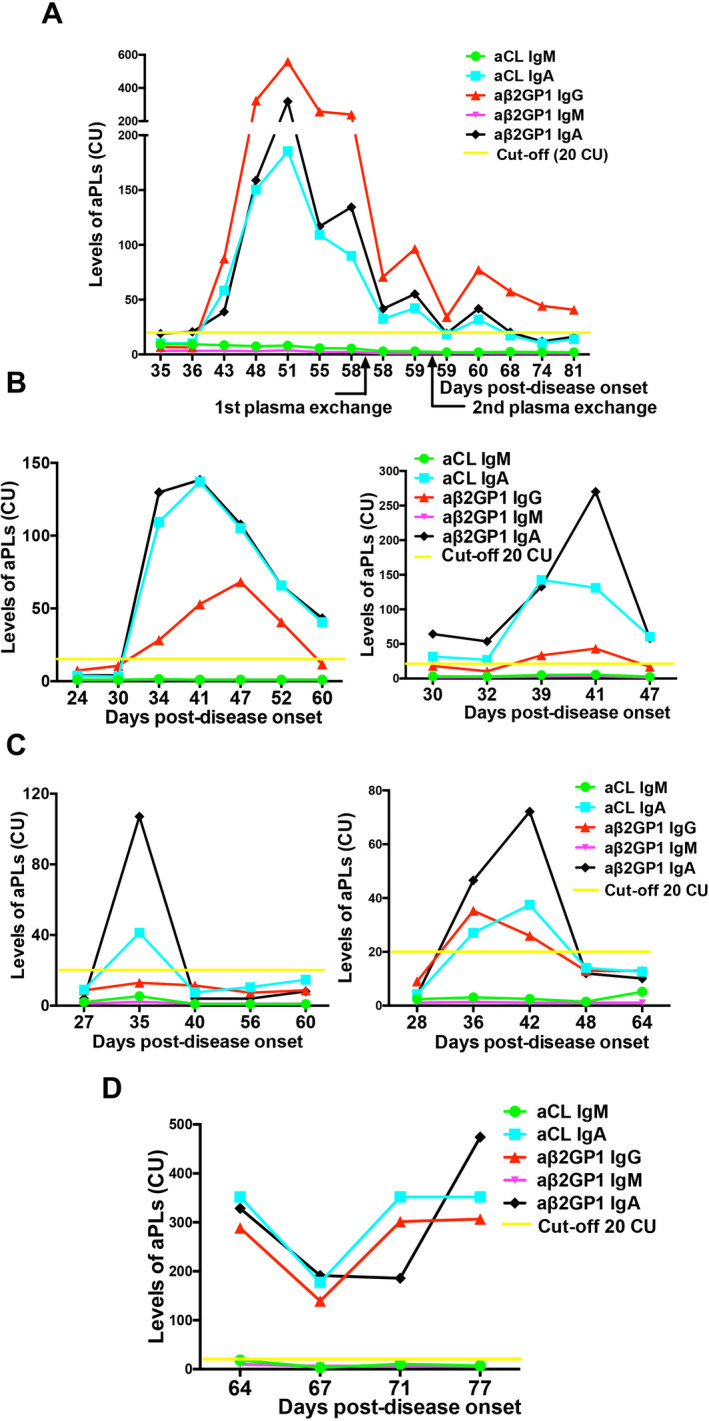
Dynamic changes in the levels of antiphospholipid antibodies (aPLs) during coronavirus disease 2019 (COVID‐19) infection in 6 critically ill patients. A, Medium levels of IgG anti–β2‐glycoprotein I (aβ2GP1) persisted after a transient appearance of IgA anti‐β2GPI + IgA anticardiolipin antibodies (aCLs) in patient 1. B, Medium levels of IgA anti‐β2GPI + IgA aCLs persisted after a transient appearance of IgG anti‐β2GPI in patient 2 (left) and patient 3 (right). C, Transient appearance of aPLs in patient 4 (left) and patient 5 (right) was observed. D, High levels of IgA aCL + IgA anti‐β2GPI + IgG anti‐β2GPI persisted in patient 6. CU = chemiluminescent units.
Last, we assessed the clinical relevance of aPLs in critically ill patients with COVID‐19 (Table 1). Mounting evidence suggests that positivity for multiple aPLs or having moderate to high titers of aPLs is more useful in predicting the possibility of cerebral infarction in COVID‐19 patients compared to positivity for a single aPL or low titers of multiple aPLs. We divided the group of patients who were positive for aPLs into the following subcohorts: 1) a single/multiplelow group (patients positive who were positive for a single aPL or positive for at least 1 aPL with low titers of all aPLs [≤40 chemiluminescent units], as previously described [12]) and 2) a multiplemedium/high group (patients who were positive for at least 1 aPL and had moderate levels [>40 chemiluminescent units] of the detected aPLs. The 3 groups consisting of critically ill patients had similar clinical and laboratory features, but the multiple medium/high group had a significantly higher incidence of cerebral infarction compared to the group of patients who were negative for aPLs (0% versus 33.3%) (P = 0.023), suggesting that aPLs (both numbers and titers) may be helpful in predicting the occurrence of cerebral infarction in COVID‐19.
DISCUSSION
The full spectrum of COVID‐19 is still under intense investigation, but increasing evidence suggests that most critically ill patients experience coagulopathy (1, 2, 3). Antiphospholipid antibodies have been considered to be one of the mechanisms leading to a proinflammatory and hypercoagulable state. In the present study, we found that aPLs were present in a substantial number of critically ill patients with COVID‐19. Although it remains unclear whether aPLs contribute to the hypercoagulable state in COVID‐19, our findings suggest the possibility that aPLs may be implicated in this process.
Infection‐induced aPL production has been widely acknowledged (13, 14). Of particular interest is the fact that we found IgA, an isotype found to be specific to mucosal immunity, as the most common isotype of the aPLs assessed. As COVID‐19 mainly affects pulmonary and intestinal mucosa, the preferential production of the IgA isotype may be associated with the breakage of mucosal immune tolerance. IgA anti‐β2GPI antibodies preferentially target the C‐terminal portion of β2GPI (domains 4 and 5) (15). Thus, the presence of IgA aPLs may suggest a novel subgroup of clinically relevant APS in critically ill COVID‐19 patients. Interestingly, we found that although IgA aCLs and IgA anti‐β2GPI antibodies transiently appeared in a subgroup of patients, they also persisted in other subgroups of patients. Unfortunately, we could not perform long‐term follow‐up of the patients evaluated in the present study. A prospective evaluation of the aPLs observed in COVID‐19 patients in the present study is needed in order to investigate whether these antibodies are persistently present and/or pathogenic in patients with COVID‐19, and whether long‐term anticoagulant therapy may be required.
While it remains unclear whether IgA aPLs are pathogenic in APS, in vivo mouse studies have demonstrated that IgA anti‐β2GPI induced significantly larger thrombi and higher tissue factor levels compared to controls (16). IgA anti‐β2GPI antibodies are significantly and independently associated with arterial thrombosis and with all thromboses in patients with systemic lupus erythematosus and APS (16). In addition, the presence of IgA anti‐β2GPI has been described as an independent risk factor for acute myocardial infarction (15, 17) and acute cerebral ischemia (18). In the present study, we found that patients with multiple aPLs, including IgA and IgG anti‐β2GPI and IgA and IgG aCLs, displayed significantly higher incidence of cerebral infarction. Unfortunately, due to the critical condition of those patients as well as the limitation of the isolation ward, a large number of patients could not be screened by ultrasound, and therefore many thrombotic events may be underrepresented. It is also worth mentioning that the patients who developed cerebral infarction may have already had atherosclerosis and aPLs. It would be of great interest to assess whether the detection of medium to high levels of multiple aPLs may help in identifying critically ill patients with COVID‐19 at risk of developing cerebral infarction in future studies.
This study has several limitations. Due to the retrospective nature of this study, in the analysis of aPLs at each time point, we only had 1 time point for some of the patients, whereas for other patients, although we had more than 1 time point, there was already positivity for aPLs at the early time point. Thus, data from only 10 patients were assessed in the analyses. The small sample size may make the present study subject to potential analytical bias. Further prospective studies on the time point at which aPLs emerge after disease onset are needed.
In conclusion, the clinical significance of aPLs in critically ill patients with COVID‐19 remains to be determined. In some patients, transient rises in aPLs may be accompanied by thrombotic complications (14). It is important to note that, although in some patients, these antibodies may be transient and disappear within a few weeks, in other genetically predisposed patients, COVID‐19 may trigger the development of “COVID‐19–induced APS‐like–syndrome.” Long‐term follow‐up of COVID‐19 patients who are positive for aPLs would be beneficial to the overall body of research investigating the effects of COVID‐19 during active disease as well as the possible long‐term outcomes of this novel coronavirus.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yongzhe Li had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Xiao, Yan Zhang, Shulan Zhang, Y. Li, Shuyang Zhang.
Acquisition of data
Xiao, Yan Zhang, X. Qin, Xia, Cao, Jiang, H. Chen, Ding, H. Zhao, Hongmin Zhang, C. Wang, J. Zhao, Sun, Tian, W. Wu, D. Wu, Ma, Y. Chen, Dong Zhang, Xie, Yan, Xiang Zhou, Liu, J. Wang, Bin Du, Y. Qin, Gao, Lu, Hou, X. Wu, Zhu, Xu, Wen Zhang, T. Li, Fengchun Zhang, Y. Zhao.
Analysis and interpretation of data
Xiao, Yan Zhang, Shulan Zhang, Shuyang Zhang.
Supporting information
Table S1
Dr. Shulan Zhang’s work is supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (grant 2017‐I2M‐3‐001). Dr. Yongzhe Li’s work is supported by the CAMS Innovation Fund for Medical Sciences (grants 2017‐I2M‐3‐001 and 2017‐I2M‐B&R‐01) and the National Natural Science Foundation of China (grants 81671618 and 81871302). Dr. Shuyang Zhang’s work is supported by CAMS Innovation Fund for Medical Sciences (grant 2020‐I2M‐CoV19‐001).
Drs. Xiao, Yan Zhang, Shulan Zhang, and X. Qin contributed equally to this work.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect 2020;9:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med 2020;58:1116–20. [DOI] [PubMed] [Google Scholar]
- 4. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, da Silva LF, de Oliveira EP, Saldiva PH, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19 [letter]. J Thromb Haemost 2020;18:1517–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up [review]. J Am Coll Cardiol 2020;75:2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS‐CoV‐2 patients: an urgent call for ultrasound screening [letter]. Intensive Care Med 2020;46:1121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 8. Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of "non‐criteria" clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features [review]. Autoimmun Rev 2015;14:401–14. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang S, Wu Z, Chen S, Li J, Wen X, Li L, et al. Evaluation of the diagnostic potential of antibodies to β2‐glycoprotein 1 domain 1 in Chinese patients with antiphospholipid syndrome. Sci Rep 2016;6:23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang S, Wu Z, Zhang W, Zhao J, Norman GL, Zeng X, et al. Antibodies to phosphatidylserine/prothrombin (aPS/PT) enhanced the diagnostic performance in Chinese patients with antiphospholipid syndrome. Clin Chem Lab Med 2018;56:939–46. [DOI] [PubMed] [Google Scholar]
- 12. Ordi‐Ros J, Saez‐Comet L, Perez‐Conesa M, Vidal X, Riera‐Mestre A, Castro‐Salomo A, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med 2019;171:685–94. [DOI] [PubMed] [Google Scholar]
- 13. Mendoza‐Pinto C, Garcia‐Carrasco M, Cervera R. Role of infectious diseases in the antiphospholipid syndrome (including its catastrophic variant) [review]. Curr Rheumatol Rep 2018;20:62. [DOI] [PubMed] [Google Scholar]
- 14. Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis 2003;62:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andreoli L, Fredi M, Nalli C, Piantoni S, Reggia R, Dall'Ara F, et al. Clinical significance of IgA anti‐cardiolipin and IgA anti‐β2glycoprotein I antibodies. Curr Rheumatol Rep 2013;15:343. [DOI] [PubMed] [Google Scholar]
- 16. Murthy V, Willis R, Romay‐Penabad Z, Ruiz‐Limon P, Martinez‐Martinez LA, Jatwani S, et al. Value of isolated IgA anti–β2 ‐glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum 2013;65:3186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ranzolin A, Bohn JM, Norman GL, Manenti E, Bodanese LC, von Muhlen CA, et al. Anti‐β2‐glycoprotein I antibodies as risk factors for acute myocardial infarction. Arq Bras Cardiol 2004;83:141–4. [DOI] [PubMed] [Google Scholar]
- 18. Staub HL, Norman GL, Crowther T, da Cunha VR, Polanczyk A, Bohn JM, et al. Antibodies to the atherosclerotic plaque components β2‐glycoprotein I and heat‐shock proteins as risk factors for acute cerebral ischemia. Arq Neuropsiquiatr 2003;61:757–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


