To the Editor,
The coronavirus disease of 2019 (COVID‐19) pandemic has created new challenges and magnified existing ones for immunocompromised individuals who may be at risk for worse clinical outcomes. Severe COVID‐19 has been associated with a hyperimmune response characterized by a surge in cytokine release described as a cytokine release syndrome (CRS). 1 Among immunocompromised patients, the inability to mount an immune response may be protective against a poor outcome.
For individuals living in homeless shelters, the lack of testing and assistance can contribute to a rapid spread of new outbreaks, which may limit a region's ability to control the pandemic. 2 In this report, we describe the clinical course of two homeless patients with a history of acquired immune deficiency syndrome (AIDS) who were admitted for COVID‐19. The details of their immunological profile and in‐hospital outcomes are described and the pathophysiological consequences of immune dysfunction in the setting of COVID‐19 discussed.
Patient 1 is a 51‐year‐old homeless male with a previous diagnosis of AIDS, not on antiretroviral therapy (ART), polysubstance abuse who had presented to the emergency department due to alcohol intoxication. He had denied any fevers, cough, or dyspnea before his presentation after he was initially stabilized. His vital signs were stable upon presentation except for an oxygen saturation of 86% on ambient air for which he was transitioned to nasal canula for oxygen therapy but escalated to 15 L of non‐rebreather to maintain an oxygen saturation >92%. Chest imaging showed bilateral infiltrates on chest x‐ray and ground glass opacities on computed tomography. A nasopharyngeal swab was positive for the novel severe acute respiratory coronavirus (SARS‐CoV‐2) (Figure 1). Sputum cultures showed concomitant infection with Streptococcus pneumonia. His detailed clinical course is given in Table 1. The patient reported a nadir CD4 cell count of 10.2 cells per uL and a corresponding HIV RNA PCR of 26 900 copies per mL. Over the course of his stay, he mounted a maximum temperature of 100.8°F on hospital day 3. He was managed conservatively with supportive oxygen therapy, azithromycin, and piperacillin tazobactam for the superimposed infection. He was not started on either chloroquine or hydroxychloroquine due to an increased risk of arrythmia with combination therapy with azithromycin. He was discharged to a shelter uneventfully on hospital day 10.
Figure 1.
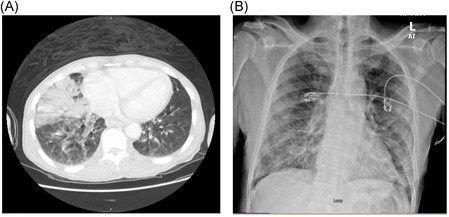
Computed tomography chest imaging showing a right lung middle lobe consolidation with bilateral ground glass opacity (A) in patient 1 and an anterior posterior chest X‐ray (B) showing bilateral lower lobe infiltrates
Table 1.
Baseline demographic, clinical, and treatment characteristics of patients
| Variable | Patient 1 | Patient 2 |
|---|---|---|
| Age, y | 51 | 63 |
| Sex | M | M |
| BMI, kg/m2 | 22 | 18 |
| Race | AA | AA |
| Symptoms at presentation | ||
| Fever | No | No |
| Dyspnea | Yes | Yes |
| Cough | No | Yes |
| Nausea/emesis | Yes | No |
| Myalgias | Yes | No |
| Vital signs baseline (peak) | ||
| Temp | 98.8 (100.8) | 98.3 (98.7) |
| RR, per min | 17 (25) | 17 (20) |
| HR, per min | 66 (99) | 94 (110) |
| Oxygen Saturation | 86 (85) | 90 (90) |
| SBP, mm Hg | 153 (180) | 130 (143) |
| Baseline (peak) lab. values | ||
| WBC, ×109/L | 5.1 (11) | 2.4 (5.7) |
| Lymphocytes, ×109/L | 18 | 34 (49) |
| Hemoglobin, g/dL | 12.7 (12.9) | 12.8 (13) |
| Platelets, ×109/L | 88 (155) | 454 (459) |
| AST, units/L | 108 (131) | 53 |
| ALT, units/L | 78 (84) | 43 |
| ALP, IU/L | 135 (135) | 92 |
| BUN, mg/dL | 11 (24) | 33 |
| Creatinine, mg/dL | 0.8 (0.9) | 1.6 |
| CRP, mg/L | … | 4.95 (4.95) |
| ESR, h | … | 131 (131) |
| Ferritin, ng/mL | 742 (1264) | 2292 (2292) |
| Superimposed infection | Streptococcus pneumoniae | None |
| HAART regimen | None | None |
| Immunological profile | ||
| CD4 cell count | 10.2 | 116.3 |
| HIV RNA PCR copies | 26 900 | 2 540 000 |
| Management | ||
| Hydroxychloroquine | NA | NA |
| Corticosteroids | NA | NA |
| Tocilizumab | NA | NA |
| Antibiotics | Azithromycin, bactrim, zosyn | Bactrim |
| Other | … | Zinc sulfate, vitamin C |
| Outcomes | ||
| Length of hospitalization, d | 10 | 7 |
| ICU admission, n (%) | 0 (0) | 0 (0) |
| Mechanical ventilation, n (%) | 0 (0) | 0 (0) |
| In‐hospital death, n (%) | 0 (0) | 0 (0) |
| Readmission | 0 (0) | 0 (0) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, Body mass index; BUN, blood urea nitrogen; CD4, cluster of differentiation 4; CRP, C reactive protein; HAART, highly active antiretroviral therapy; HIV RNA PCR, human immunodeficiency virus ribonucleic acid polymerase chain reaction; HR, heart rate; ICU, intensive care unit; RR, respiratory rate; SBP, systolic blood pressure; SR, erythrocyte sedimentation rate; WBC, white blood cell count.
Patient 2 is another homeless male, aged 63 years, with a previous history of AIDS, not on ART, hypertension and hyperlipidemia who presented with a 3‐day duration of worsening nonproductive cough, subjective fevers, myalgias, and dyspnea. He self‐reported exposure to individuals who had been previously diagnosed with SARS‐CoV‐2 infection. A nasopharyngeal swab specimen was consistent with the SARS‐CoV‐2 infection. Upon presentation to the emergency room, he was afebrile and hemodynamically stable saturating at 93% ambient air. Further details on his clinical presentation including baseline and peak laboratory findings, and imaging characteristics are given in Table 1 and Figure 1, respectively. His immunological profile showed a CD4 cell count of 116/uL, and a HIV RNA PCR viral load of 2 540 000 copies per mL. He received supportive treatment with nasal canula oxygen therapy, acetaminophen for headaches and antibiotic treatment for the pneumonia. Sputum cultures were negative for other infectious etiology of pneumonia. The patient was discharged to a homeless shelter on hospital day 7 with no COVID‐19‐associated complications.
Although limited by few studies and case series, the current evidence regarding the association between HIV and COVID‐19 shows that people with HIV are not at an increased risk for worse outcomes. 3 , 4 , 5 , 6 , 7 The poor clinical outcomes in COVID‐19 have been associated with laboratory features of CRS. These include abnormal levels of inflammatory cytokines (IL‐6, IL‐10, IL‐2, and IFN‐γ) and a decrease in CD4 and CD8 cells. 1 In chronic untreated HIV infection, progression to AIDS is inevitable and plasma levels of inflammatory mediators (IL‐6, IFN‐γ, and TNF) are typically elevated. 8 The reason for this sustained elevation in cytokines is unclear; however, it is likely that a chronic hyperinflammatory state may serve a protective role against COVID‐19‐related complications. A better understanding of ways in which these mediators may drive immune homeostasis is key to the management of these individuals. In both patients, the initiation of ART was withheld during their acute state of infection with SARS‐CoV‐2. Instead, ART was initiated weeks after their successful discharge to a homeless shelter.
In conclusion, we describe our experience with AIDS and COVID‐19 in two homeless patients who were expected to be at an increased risk for worse outcomes. Despite their poor immunological profile, their clinical course was uncomplicated, and both were successfully discharged alive with scheduled followup and monitoring.
REFERENCES
- 1. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID 19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus is spreading under the radar in US homeless shelters . https://www.nature.com/articles/d41586-020-01389-3#ref-CR1. Accessed June 26, 2020. [DOI] [PubMed]
- 3. Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. COVID‐19 pneumonia in patients with HIV—a case series [published online ahead of print May 28, 2020]. J Acquir Immune Defic Syndr. 10.1097/QAI.0000000000002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruan L, Zhang Y, Luo Y. Clinical features, and outcomes of four HIV patients with COVID‐19 in Wuhan, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toombs JM, Van den Abbeele K, Democratis J, Merricks R, Mandal AKJ. Missouris CG COVID‐19 in 3 people living with HIV in the United Kingdom. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Ma Q, Wang X, Tang M, Lin J, Xiao B. The characteristics of two patients coinfected with SARS‐CoV‐2 and HIV in Wuhan, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adadi P, Kanwugu ON. Living with HIV in the time of COVID‐19: a glimpse of hope. J Med Virol. 2020. 10.1002/jmv.26118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Funderburg NT, Andrade A, Chan ES, Lu D, Rosenkranz SL, Clagett B. Dynamics of immune reconstitution and activation markers in HIV+ treatment‐naïve patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLOS One. 2013;8(12):e83514. [DOI] [PMC free article] [PubMed] [Google Scholar]


