Abstract
This study investigates the clinical and imaging characteristics of coronavirus disease 2019 (COVID‐19) patients with false‐negative nucleic acids. Mild‐to‐moderate COVID‐19 patients, including 19 cases of nucleic acid false‐negative patients and 31 cases of nucleic acid positive patients, were enrolled. Their epidemiological, clinical, and laboratory examination data and imaging characteristics were analyzed. Risk factors for false negatives were discussed. Compared with the nucleic acid positive group, the false‐negative group had less epidemiological exposure (52.6% vs 83.9%; P = .025), less chest discomfort (5.3% vs 32.3%; P = .035), and faster recovery (10 [8, 13] vs 15 [11, 18.5] days; P = .005). The number of involved lung lobes was (2 [1, 2.5] vs 3 [2, 4] days; P = .004), and the lung damage severity score was (3 [2.5, 4.5] vs 5 [4, 9] days; P = .007), which was lighter in the nucleic acid false‐negative group. Thus, the absence of epidemiological exposure may be a potential risk factor for false‐negative nucleic acids. The false‐negative cases of COVID‐19 are worth noting because they have a risk of viral transmission without positive test results, lighter clinical manifestations, and less history of epidemiological exposure.
Keywords: clinical characteristics, false negative SARS‐CoV‐2 nucleic acid, mild‐to‐moderate COVID‐19, risk factors
Highlights
The clinical and imaging features of mild‐to‐moderate COVID‐19 patients with nucleic acid false negative have certain characteristics. These patients have less clear history of epidemiological exposure, lighter clinical symptoms, lower lung damage severity score, and laboratory test results are better than those with nucleic acid positive.
1. INTRODUCTION
The outbreak of coronavirus Disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has become a major public health incident of worldwide concern. As of 20 April 2020, there have been nearly 2.6 million cases of infection in 200 countries and regions around the world. The SARS‐CoV‐2 infection has a latent period and is highly contagious. Its spread is much higher than the Middle East respiratory syndrome‐Coronavirus and SARS‐CoV. 1 Therefore, timely identification of COVID‐19 cases is critical for controlling the pandemic. Currently, the SARS‐CoV‐2 real‐time polymerase chain reaction (RT‐PCR) test is the gold standard for diagnosing COVID‐19. However, clinical studies have shown that many cases present typical clinical symptoms and lung imaging changes, but their RT‐PCR test results for SARS‐CoV‐2 are negative. 2 , 3 , 4 These cases can easily become loopholes in the prevention and control of the COVID‐19 pandemic. Recent studies have shown that SARS‐CoV‐2 nucleic acid detection combined with specific antibody detection significantly improves the diagnostic accuracy of COVID‐19. 5 , 6 , 7 , 8 However, due to the outbreak of the COVID‐19 pandemic, the number of patients has grown rapidly. In this setting, there is an insufficient supply of SARS‐CoV‐2 nucleic acid detection kits or antibody detection kits. Some countries only give priority to testing high‐risk or critically ill patients, that is, patients with a clear history of epidemiological exposure, or clinical and imaging characteristics of suspected COVID‐19. Therefore, understanding the clinical and imaging characteristics of nucleic acid false‐negative COVID‐19 patients is of great significance for their timely identification and reducing the spread of epidemics due to missed diagnosis.
Some studies have reported the clinical and imaging characteristics of COVID‐19 patients positive for SARS‐CoV‐2 nucleic acid, 9 , 10 , 11 , 12 but there are limited reports on those negative for SARS‐CoV‐2 nucleic acid. In addition, the differences in epidemiology, clinical manifestations, and imaging characteristics between COVID‐19 patients with false‐negative nucleic acid results and those with positive nucleic acid results are not clear. In this study, we retrospectively analyzed and compared the detailed clinical data of mild‐to‐moderate COVID‐19 patients with positive or negative results for SARS‐CoV‐2 nucleic acid, who were hospitalized in the Hezheng Ward of Shenzhen Hospital of Southern Medical University. All the patients were diagnosed according to the latest diagnosis and treatment plan. 13 Meanwhile, the possible risk factors of a nucleic acid false negative in COVID‐19 patients were analyzed.
2. MATERIALS AND METHODS
2.1. Study design and patients
This is a retrospective analysis. We included COVID‐19 patients who were hospitalized in Hezheng Ward of Shenzhen Hospital of Southern Medical University from 3 February 2020 to 7 March 2020. All included patients who underwent SARS‐CoV‐2 nucleic acid detection, specific IgM and IgG antibody detection, and lung CT examination. There were no exclusion criteria for the patients. According National Health Commission of the People's Republic of China Diagnostic and Treatment Protocol for COVID‐19 (trial Seventh Edition), 13 the definition criteria for COVID‐19 suspected case were (a) The patient had a clear history of epidemiological exposure and had at least two of the following clinical manifestations and (b) the patient had no history of clear epidemiological exposure and had three of the following clinical manifestations. The clinical manifestations included (a) fever and/or respiratory symptoms, (b) imaging features of viral pneumonia, and (c) the number of white blood cells in the early stage of the disease was normal or decreased, and the number of lymphocytes was normal or decreased. In addition, "clear epidemiological exposure" was defined as follows: a history of contact with a virus‐infected person (nucleic acid‐positive) within 14 days before the onset of illness, or a history of travel or residence in Wuhan and surrounding areas. The definition criteria of COVID‐19 confirmed cases were suspected cases with at least one of the following etiological or serological evidence, including (a) RT‐PCR test positive for SARS‐CoV‐2 nucleic acid, (b) The sequence of the isolated virus was highly homologous to SARS‐CoV‐2 and (c) Serum specific IgM or IgG antibody for SARS‐CoV‐2 was positive. Clinical classification criteria included (a) Mild COVID‐19, in which the symptoms included fever and mild respiratory symptoms, but there were no signs of pneumonia on lung imaging. (b) Moderate COVID‐19, in which the symptoms included fever and cough and other respiratory symptoms, and lung imaging showed viral pneumonia. However, the vital signs were stable, and the blood oxygen saturation was more than 93% without oxygen support. In the end, 50 eligible cases of mild or moderate COVID‐19 were included in the study. According to RT‐PCR results, we divided the cases into two groups of the nucleic acid positive group and nucleic acid false‐negative group. In the nucleic acid positive group, all cases had positive RT‐PCR results for SARS‐CoV‐2 of nasal or pharyngeal swabs. In the nucleic acid false‐negative group, all cases had clinical features of suspected cases of COVID‐19 and positive specific IgM/IgG antibody, but their nucleic acid detection of nasal, pharyngeal and anal swabs was continuously negative from the initial screening to hospitalization. This study was approved by the Ethics Committee of Shenzhen Hospital, Southern Medical University (NYSZYYEC20200013). The data were anonymous and the informed consent was therefore waived.
2.2. Data collection
All data are independently reviewed by two investigators (Yan Rong and Xinhua Liang) to verify the accuracy of the data. The collected data included demographic data, epidemiological exposure history, chronic comorbidities, disease diagnosis and treatment, clinical symptoms, laboratory tests, and chest CT scan results.
The CT images of all patients were reviewed and evaluated again by a professor of imaging of the SARS‐CoV‐2 Expert Group (WJ) in our hospital in a blinded manner. The evaluation included four aspects: (a) the affected lung lobe, (b) the lesion range score of the affected lung lobe was evaluated based on lesion diameter, which was as follows: 0 point (no lesion), 1 point (diameter < 1 cm), 2 points (diameter 1 to <3 cm), 3 points (diameter from 3 cm to <50% of the lobe), and 4 points (50%‐100% of the lobe), (c) the severity of lung lesions according to the number of affected lung lobe and the lesion range of each involved lobe, and (d) the imaging manifestations of the lung lesions.
All patients' nasal or pharyngeal swabs for SARS‐CoV‐2 RT‐PCR detection before admission were performed by kits provided by six companies (DAAN, Sansure Biotech, BGI, Shanghai ZJ Biotech, Geneodx, Biogerm). All positive results were reviewed and confirmed at the Shenzhen Center for Disease Control and Prevention (CDC). After admission, all patients underwent nasal and pharyngeal swab nucleic acid detection every 3 to 5 days and later received anal swab nucleic acid detection. Blood samples were collected during hospitalization.
Specific IgM/IgG antibodies for SARS‐CoV‐2 were detecting with time‐resolved fluorescence immunochromatography using SARS‐CoV‐2 specific IgM/IgG antibody kit (Beijing Digret Biotechnology Co., Ltd., Beijing, China).
2.3. Statistical analysis
All data were statistically analyzed using SPSS 25.0. Categorical variables, presented as n (%), were analyzed with χ 2 or Fisher exact tests. The continuous variables of non‐normal distribution were expressed as median (25%, 75%) and compared with the Mann‐Whitney U test. Univariate and multivariate logistic binary regression analysis was used to analyze risk factors. A P < .05 is considered statistically significant.
3. RESULTS
3.1. Results of nucleic acid detection and antibody detection of the included patients
According to the grouping criteria, 31 of the 50 mild‐to‐moderate COVID‐19 cases who were positive for SARS‐CoV‐2 RT‐PCR of nasal and pharyngeal swabs, were included in the nucleic acid positive group. Among them, 12.9% (4/31) were single IgM positive, 22.58% (7/31) were single IgG positive, 61.29% (19/31) were double positive of IgM and IgG, and only 3.23% (1/31) were double negative of IgM and IgG (Figure 1A). The remaining 19 cases whose nucleic acid test was continuously negative were included in the nucleic acid false‐negative group, among which, 52.63% (10/19) were single IgM positive, and 47.37% (9/19) were double positive of IgG and IgM (Figure 1B). There was no single IgG positive case in this group. The median days of antibody detection after symptom onset were 25 (19, 29) days in the nucleic acid positive group and 10 (7, 14) days in the nucleic acid negative group.
Figure 1.
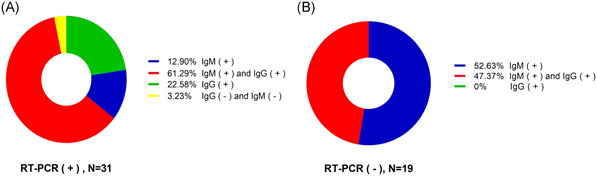
Composition of the study cohort. Fifty cases of mild‐to‐moderate COVID‐19 were tested for SARS‐CoV‐2 nucleic acid and specific antibody. A, Result of specific antibody detection in the nucleic acid positive group (n = 31). B, Result of specific antibody detection in the false‐negative group (n = 19). COVID‐19, coronavirus disease 2019; RT‐PCR, real‐time polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
3.2. Comparison of clinical characteristics between nucleic acid false‐negative group and nucleic acid positive group
The clinical characteristics of the two groups were compared (Table 1). In the nucleic acid false‐negative group, the median age was 31 (range 25‐38), for which the females accounted for 78.9% (15/19). And 52.6% (10/19) had epidemiological exposure such as Hubei residence or contact with confirmed cases. Chronic comorbidities accounted for 31.6% (6/19). We also found moderate COVID‐19 cases accounted for 90% (17/19), while mild cases only accounted for 10%. Their main clinical manifestations were fever (14/19, 73.7%), dry cough (13/19, 68.4%), and throat discomfort (8/19, 42.1%). The median time from the onset of symptoms to the first visit was 1 day (1, 3.5).
Table 1.
The clinical characteristics of the study cohort
| Items | Patients (n = 50) | Nucleic acid positive (n = 31) | Nucleic acid false negative (n = 19) | P value |
|---|---|---|---|---|
| Age (y) | 34 (25–42) | 35 (25–44) | 31 (25–38) | .542 |
| <18 | 8 (16%) | 4 (12.9%) | 4 (21.05%) | .595 |
| 19‐40 | 28 (56%) | 17 (54.8%) | 11 (57.9%) | |
| >40 | 14 (28%) | 10 (32.3%) | 4 (21.05%) | |
| Sex | ||||
| Male | 17 (34%) | 13 (41.9%) | 4 (21.1%) | .218 |
| Female | 33 (66%) | 18 (58.1%) | 15 (78.9%) | |
| Clinical type | ||||
| Mild COVID‐19 | 10 (20%) | 8 (25.8%) | 2 (10.1%) | .282 |
| Moderate COVID‐19 | 40 (80%) | 23 (74.2%) | 17 (89.5%) | |
| Exposure history | ||||
| Yes | 36 (72%) | 26 (83.9%) | 10 (52.6%) | .025 |
| No | 14 (28%) | 5 (16.1%) | 9 (47.4%) | |
| Chronic underlying disease | ||||
| Chronic lung disease | 4 (8%) | 2 (6.5%) | 2 (10.5%) | .629 |
| High blood pressure | 1 (2%) | 0 (0%) | 1 (5.3%) | .38 |
| Postoperative tumor | 2 (4%) | 2 (6.5%) | 0 (0%) | .519 |
| Diabetes | 1 (2%) | 1 (3.2%) | 0 (0%) | 1 |
| Chronic kidney disease | 1 (2%) | 0 (0%) | 1 (5.3%) | .38 |
| Allergic rhinitis | 2 (4%) | 0 (0%) | 2 (10.5%) | .14 |
| Hyperlipidemia | 1 (2%) | 1 (3.2%) | 0 (0%) | 1 |
| Total number of patients with comorbidities | 12 (24%) | 6 (19.4%) | 6 (31.6%) | .496 |
| Signs and symptoms | ||||
| Fever | 33 (66%) | 19 (61.3%) | 14 (73.7%) | .540 |
| Cough | 35 (70%) | 22 (71.0%) | 13 (68.4%) | 1 |
| Expectoration | 4 (8%) | 2 (6.5%) | 2 (10.5.%) | .629 |
| Sore throat | 13 (26%) | 5 (16.1%) | 8 (42.1%) | .54 |
| Chest pain, Chest distress, breathlessness | 11 (22%) | 10 (32.3%) | 1 (5.3%) | .035 |
| Muscle aches | 5 (10%) | 2 (6.5%) | 3 (15.8%) | .355 |
| Fatigue | 3 (6%) | 2 (6.5%) | 1 (5.3%) | 1 |
| Gastrointestinal symptoms | 4 (8%) | 4 (12.9%) | 0 (0%) | .284 |
| Headache and dizziness | 5 (10%) | 3 (5.9%) | 2 (10.5%) | 1 |
| Chills | 3 (6%) | 1 (3.2%) | 2 (10.5%) | .549 |
| Runny nose | 3 (6%) | 1 (3.2%) | 2 (10.5%) | .549 |
| Time interval from symptom onset to first visit (d) | 2 (1, 4) | 2 (1, 4) | 1 (1, 3.5) | .926 |
| Clinical remission time (d) | 12.5 (10, 16) | 15 (11, 18.5) | 10 (8, 13) | .005 |
| Hospitalization days (d) | 19.5 (15, 24) | 23 (18, 27) | 15 (13, 18) | P < .001 |
The nucleic acid false‐negative group had fewer epidemiological exposure history (52.6% vs 83.9%; P = .025). There was no significant difference in chronic comorbidities between the two groups (19.4% vs 31.6%, P = .496). The symptoms of chest tightness and chest discomfort in the nucleic acid false‐negative group were less than those in the nucleic acid positive group (5.3% vs 32.3%; P = .035) (Table 1). The other symptoms were similar between the two groups. The hospitalization days of the nucleic acid false‐negative group were 15 (13, 18) days, significantly shorter than those of the nucleic acid positive group (23 (18, 27) days) (P < .01). Meanwhile, the clinical remission time of the nucleic acid false‐negative group was also significantly shorter than that (10 vs 15 days, P = .005). The liver function, kidney function, and myocardial enzyme analysis of the two groups of patients were basically normal (data not shown). The results of the blood routine test of both groups were within the normal range (Figure 2A‐E). The median absolute value of lymphocytes in the nucleic acid positive group was lower than the nucleic acid false‐negative group (0.99 × 109 vs 1.34 × 09/L, P = .037) (Figure 2C). The median of hypersensitive C‐reactive protein increased slightly (Figure 2E). The overall condition of the nucleic acid false‐negative group was lighter than that of the nucleic acid positive group.
Figure 2.
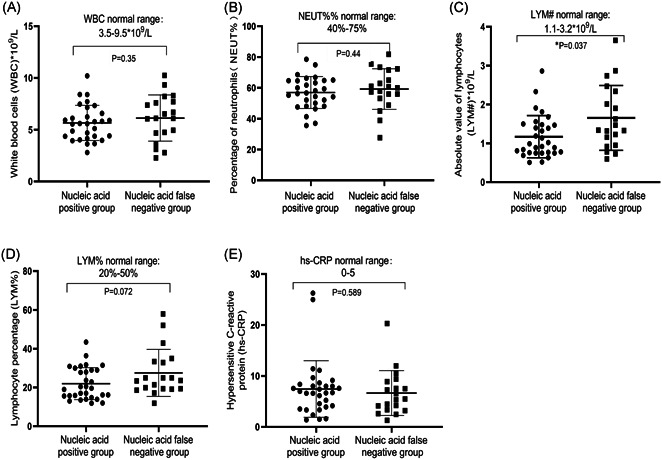
Comparison of blood routine results and hs‐CRP between the nucleic acid positive group and nucleic acid false‐negative group. A, Total number of white blood cells; B, percentage of neutrophils; C, the absolute number of lymphocytes; D, percentage of lymphocytes; E, hypersensitive C‐reactive protein in the nucleic acid positive group and nucleic acid false negative group
3.3. Comparison of lung imaging between the nucleic acid false negative group and nucleic acid positive group
Single lung lobe involvement was most common in the nucleic acid false‐negative group, accounting for 41.2%. In the nucleic acid positive group, multiple lung lobe involvement accounted for the majority. Among them, involvements of four lung lobes accounted for 26%, and 13% of patients had involvement of five lung lobes. Statistically, the median number of lung lobe involvement in the nucleic acid false‐negative group was significantly lower than the nucleic acid positive group ((2 [1, 2.5] vs 3 [2, 4]; P = .004) (Figure 3A). In the nucleic acid false‐negative group, the percentage of cases with lesion range score of 1 was higher than that in the nucleic acid positive group (35.5% vs 26.1%). However, the percentage of cases with lesion range score of 2 (38.7% vs 49.3%) and 3 (19.4% vs 24.6%) was less than that in the nucleic acid positive group. There was no significant difference in the median lesion range scores in the two groups (2 [1, 3] vs 2 [1, 2.5]; P = .692) (Figure 3B). After that, we comprehensively evaluated the severity of lung lesions based on the number of affected lung lobes and the range of lesions in each lung lobe. The results showed that the nucleic acid false‐negative group had less severe lesions than the nucleic acid positive group (3 [2.5, 4.5] vs 5 [4, 9]; P = .007) (Figure 3C). The imaging features of the lung lesions in the two groups were similar. The features of ground‐glass opacity and interlobular interstitial thickening accompanied by ground‐glass opacity were most commonly observed. In addition, there was one case of pleural thickening in each of the two groups, accompanied by a small amount of pleural effusion. There was no lymphadenopathy in both groups.
Figure 3.
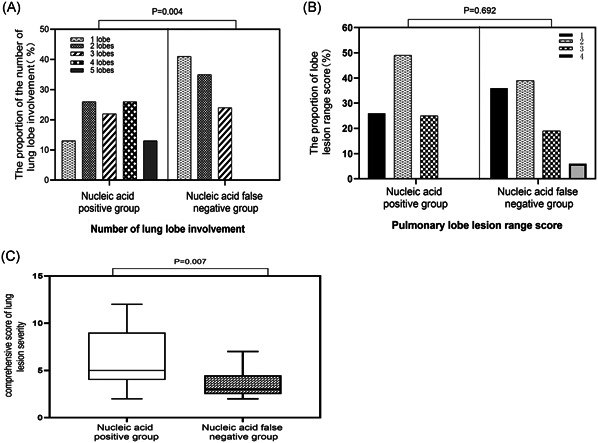
Comparison of lung lesions between the nucleic acid positive group and nucleic acid false‐negative group. A, The proportion of involved lung lobes. B, The proportion of lesion range score. C, Comprehensive score of lung damage
3.4. High‐risk factors for false‐negative SARS‐CoV‐2 nucleic acid in COVID‐19 patients
We further explored the related factors of false‐negative SARS‐CoV‐2 nucleic acid in COVID‐19 patients. Univariate logistic binary regression analysis showed that those with a clear history of epidemiological violence were not likely to have negative nucleic acid results (OR = 0.192, 95% CI: 0.051‐0.727; P = .015) (Table 2). However, when performing multivariate logistic analysis, these factors did not enter the equation, which may be related to the small sample size and the bias in the number of patients of the two groups.
Table 2.
Logistic binary regression analysis of factors related to severe acute respiratory syndrome coronavirus‐2 nucleic acid false‐negative cases
| Variable | B | SE | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Epidemiological exposure history | −1.649 | 0.679 | .015 | 0.192 | 0.051 | 0.727 |
4. DISCUSSION
Of the 31 SARS‐CoV‐2 RT‐PCR positive cases in this study, 30 cases also showed positive results for the SARS‐CoV‐2 specific antibody, indicating that the positive result of SARS‐CoV‐2 nucleic acid test is highly reliable.
As the antibody detection of the nucleic acid positive group was performed during the recovery period, the nucleic acid positive group was dominated by IgG and IgM double‐positive and IgG single positive cases. However, in the nucleic acid false‐negative group, due to antibodies were very important for the diagnosis of COVID‐19, antibodies testing was performed during the acute period, with median days of antibody detection of 10 (7, 14) days after symptom onset. According to the antibody time dynamics of COVID‐19, 5 the single IgG positive in the acute period does not meet the diagnostic criteria of COVID‐19. Thus, there was no IgG single positive case in the nucleic acid false‐negative group.
However, the number of COVID‐19 patients with false‐negative SARS‐CoV‐2 nucleic acid results was 19 (38%), accounting for a considerable proportion. The clinical symptoms, laboratory tests, and comprehensive assessment of lung lesions in the nucleic acid false‐negative group were milder than those in the nucleic acid‐positive group. The clinical remission time was also significantly shorter in the nucleic acid false‐negative group. Because of negative nucleic acid tests, these patients are easy to be missed, which will hinder the control of the epidemic of COVID‐19. Thus, understanding the clinical features of COVID‐19 patients with false‐negative nucleic acid results and identifying them in a timely manner is of great significance for epidemic prevention and control.
The clinical and pulmonary imaging features of nucleic acid false‐negative mild‐to‐moderate COVID‐19 patients are similar to typical nucleic acid positive COVID‐19 patients. 10 , 11 , 12 Our research showed that the symptoms of chest discomfort, chest pain, and shortness of breath in the nucleic acid false‐negative group were significantly less than those in the nucleic acid positive group. The difference in the comprehensive scores of lung lesions between the two groups may be part of the reason for the significant difference in chest symptoms, but the oxygen saturation and heart rate of the two groups on admission were within the normal range and there was no significant difference. Therefore, this may be related to the anxiety of the patients in the nucleic acid positive group. COVID‐19 is a severe source of psychological stress. Due to the fear of the disease and uncertainty about the future, it will cause patients to have anxiety and stress‐related diseases. 14 In addition, the direct effect of SARS‐Cov‐2 infection on the human central nervous system can also cause neuropsychiatric symptoms. 15 Therefore, we should pay more attention to the mental and psychological problems of COVID‐19 patients. More humane care and emotional counseling should be given to COVID‐19 patients. Mental health counselors and therapists should participate in the comprehensive treatment and follow‐ups of COVID‐19 patients.
The difference in hospitalization time between the two groups was more obvious. The longer hospital stays of the nucleic acid‐positive group may partly because the Shenzhen government implemented strict isolation and control measures for confirmed COVID‐19 cases. Nucleic acid‐positive patients are managed as COVID‐19 confirmed cases, and nucleic acid false‐negative patients are considered as suspected cases of COVID‐19. The COVID‐19 confirmed patients whose nucleic acid turned negative for two consecutive times and had clinical remission were continued to be hospitalized for free and observed in isolation for 14 days. The hospitalization and isolation period of suspected cases of COVID‐19 was shorter than the nucleic acid positive group. Thus, identification of COVID‐19 patients with false‐negative nucleic acid results is conducive to strengthening the management and control of the COVID‐19 epidemic.
Although the nucleic acid false‐negative COVID‐19 patients had less severe lung lobe involvement and lung damage than the nucleic acid‐positive group, the nucleic acid false‐negative results were not significantly related to these factors. This may be because SARS‐CoV‐2 infection started in the lungs rather than the upper respiratory tract. The severity of lung lesions was not significantly related to the viral load of the upper respiratory tract. 12 To et al 16 found that the upper respiratory tract virus load was not significantly different between critical and noncritical COVID‐19 cases. In addition, Zheng et al 17 found that there was no significant difference in SARS‐CoV‐2 RNA clearance in the upper respiratory tract between the critical and noncritical patients. The viral load of the upper respiratory tract changes with time, and generally reaches a peak about a week after the onset of symptoms, and then gradually decreases, while the viral load of the lower airway monitored during the same period has no such trend. 16 Therefore, the upper airway viral load may not reflect the severity of lung lesions.
In this study, our results showed that patients without a clear history of epidemiological exposure were more likely to be falsely negative for SARS‐CoV‐2 nucleic acids, while patients with a clear history of exposure were more likely to be positive for SARS‐CoV‐2 nucleic acids. "Clear epidemiological exposure history" refers to close contact with patients with COVID‐19, or to live in high‐risk areas of Hubei and Wuhan. As with other respiratory infectious diseases, COVID‐19 patients release aerosols and droplets containing SARS‐CoV‐2 virus from coughing, sneezing, talking, and breathing, 18 , 19 which can cause airborne transmission of the virus. The range of virus transmission is mainly within 6 feet of the infected person. 20 Therefore, patients with a clear history of epidemiological exposure are exposed to an environment with higher virus load, possibly leading to positive SARS‐CoV‐2 nucleic acid results. For patients without a clear history of epidemiological exposure, the exposure to the SARS‐CoV‐2 may be transient, and the environmental viral load may also be relatively low. However, whether it is related to the upper airway viral load and therefore easier to be positive for nucleic acid tests needs further investigation.
The reason for the false‐negative of SARS‐CoV‐2 nucleic acid test is not only related to the disease itself, but also with some other factors. The viral load of the nasal and pharyngeal parts of the upper respiratory tract is different. 10 , 16 , 21 Thus, different sampling sites may also affect nucleic acid detection results. In addition, nucleic acid detection kits and standard operating procedures are also factors that may affect nucleic acid detection results. 6 , 21 Therefore, attention should be paid to the combination of multi‐site sampling 22 and the standardization of the operation process during nucleic acid detection. The epidemiological, clinical, imaging and nucleic acid and antibody test results should be combined to make the diagnosis of COVID‐19. 23
However, this study still has some limitations. For example, the sample size was relatively small. In addition, the retrospective design itself was limited. Further studies with larger sample size are needed.
In conclusion, the clinical and imaging characteristics of mild‐to‐moderate COVID‐19 patients with nucleic acid false‐negative have certain characteristics. The clinical symptoms, laboratory tests, clear history of epidemiological exposure, and comprehensive evaluation of the severity of lung disease in these patients are less severe than those with positive nucleic acids. However, because of negative results for nucleic acids, these patients are easily missed, leading to SARS‐CoV‐2 transmission. Attention should be paid to these patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 81470206) and Shenzhen Baoan District Science and Technology Innovation Bureau (No. 2017JD170).
Rong Y, Wang F, Liu J, et al. Clinical characteristics and risk factors of mild‐to‐moderate COVID‐19 patients with false negative SARS‐CoV‐2 nucleic acid. J Med Virol. 2021;93:448–455. 10.1002/jmv.26242
Contributor Information
Jing Wang, Email: wjing7997@163.com.
Yi Shi, Email: yishi_202011@163.com.
REFERENCES
- 1. Yan Y, Chang L, Wang LN. Laboratory testing of SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 (2019‐nCoV): Current status, challenges, and countermeasures. Rev Med Virol. 2020;30(3):e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortega VE, Li X, O'neal WK, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;201:540‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abbasi J. The promise and peril of antibody testing for COVID‐19. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 5. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YS, Kang H, Liu XF, Tong ZH. Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J Med Virol. 2020;92:538‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du Z, Zhu FX, Guo FZ, Yang B, Wang TB. Detection of antibodies against SARS‐CoV‐2 in patients with COVID‐19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect. 2020;80(4):394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Notice on Printing and Distributing the New Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Version 7)‐Marked Version (1).
- 14. Li W, Yang Y, Liu ZH, et al. Progression of mental health services during the COVID‐19 outbreak in China. Int J Biol Sci. 2020;16(10):1732‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Troyer EA, Kohn JN, Hong SZ. Are we facing a crashing wave of neuropsychiatric sequelae of COVID‐19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng C, Wang J, Guo H, et al. Risk‐adapted treatment strategy For COVID‐19 patients. Int J Infect Dis. 2020;94:74‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei JJ, Li YG. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9 Suppl):S102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Godri Pollitt KJ, Peccia J, Ko AI, et al. COVID‐19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum Genomics. 2020;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East Respiratory Syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63(3):363‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicastri E, D'Abramo A, Faggioni G, et al. Coronavirus disease (COVID‐19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill. 2020;25(11):2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID‐19). Eur Radiol. 2020:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


