Abstract
The situation of the coronavirus disease 2019 (COVID‐19) continues to evolve, our study explored the significance of serum levels of matrix metalloproteinase 3 (MMP3) as a marker for patients with COVID‐19. Sixty‐two COVID‐19 patients in the First Hospital of Hunan University of Chinese Medicine and Loudi Center for Diseases Prevention and Control, from January to March 2020, were sampled as the novel coronavirus pneumonia infected group. One hundred and thirty‐one cases from the First Hospital of Hunan University of Chinese Medicine, including 67 healthy individuals and 64 non‐COVID‐19 inpatients, served as the noninfected group. Approximately every 5 days, sera from 20 cases were collected and analyzed three times, using an automatic biochemical analyzer, to detect serum MMP3 concentrations. Correlation was analyzed between MMP3 and other proinflammatory cytokines. Following normality tests, differences in serum MMP3 levels between the infected and noninfected group were analyzed via SPSS (version 25.0) software, using the Wilcoxon rank sum test. The MMP3 concentration was 44.44 (23.46 ~ 72.12) ng/mL in the infected group and 32.42 (28.16 ~ 41.21) ng/mL in the noninfected group. The difference between the two groups was statistically significant (Z = −2.799, P = .005 < .05). A positive correlation was found between MMP3 and interleukin 1β (IL‐1β; r = .681, P = .000 < .05), and IL‐6 (r = .529, P = .002 < .05). Serum MMP3 concentration, measured over three separate time points, were 55.98 (30.80 ~ 75.97) ng/mL, 34.84 (0.00 ~ 51.84) ng/mL, and 5.71 (0.00 ~ 40.46) ng/mL, respectively. Detection of serum MMP3 levels may play an important role in the development of therapeutic approaches for COVID‐19 and may indicate the severity of disease.
Keywords: COVID‐19, inflammation, MMP3 concentrations
1. INTRODUCTION
A novel coronavirus, named the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by the World Health Organization, was first reported in Wuhan, China in December 2019. SARS‐CoV‐2 causes coronavirus disease 2019 (COVID‐19), and typical COVID‐19 symptoms include dry cough, fever, and fatigue. 1 , 2 Since its emergence, SARS‐CoV‐2 has spread rapidly all over the world, arousing widespread concern. To date, the viral nucleic acid test remains the main diagnostic tool used to detect COVID‐19, whereas serum immunoglobulin G (IgG) and IgM antibodies can be used to detect the SARS‐CoV‐2 infection. 3 Laboratory examination plays a vital role in the diagnosis and treatment of COVID‐19, wherein relevant test indicators may provide evidence‐based support for clinicians. Our previous study 4 has found that MMP3 has good clinical value in the differential diagnosis of benign and malignant hydrothorax and ascites. In this study, we attempt to show a possible connection between matrix metalloproteinase 3 (MMP3) and COVID‐19.
MMP3 is an important member of a large family of MMPs containing zinc‐dependent endopeptidases. Matrix degradation and remodeling have been recognized as the main function of MMPs. However, subsequent studies reveal that MMPs may participate in diverse pathophysiological processes, such as the regulation of inflammatory and immune responses as well as cell‐cell communication, among others. Reportedly, 5 , 6 in addition to the above functions, MMP3 also activates other MMPs in the family. In particular, it participates in many physiological and pathological processes that are associated with the inflammatory process. For example, studies have confirmed that MMP3 levels may be used to monitor the activity of rheumatoid arthritis and to predict its severity. 7 , 8 Recent studies have investigated the effects of MMP3 on respiratory disorders, including acute lung injury (ALI), acute respiratory distress syndrome (ARDS), pulmonary fibrosis, and lung cancer. 5 , 9 Therefore, this study makes a major contribution to research on COVID‐19 by demonstrating the significance of MMP3 and providing ideas for future scientific research.
2. MATERIALS AND METHODS
2.1. Patients
Sixty‐two Chinese patients with COVID‐19, from the First Hospital of Hunan University of Chinese Medicine and Loudi Center for Diseases Prevention and Control, who met the diagnostic criteria for COVID‐19 and the requirements of the treatment plan (7th trial edition) issued by the General Office of National Health Commission of the People's Republic of China and Office of National Administration of Traditional Chinese Medicine, 10 were included in the study as the infected group. A total of 131 cases from the First Hospital of Hunan University of Chinese Medicine, including 67 healthy individuals and 64 hospitalized patients, served as the noninfected group. Hospitalized patients are those who suffer from diabetes, hematological diseases, malignant tumors, and other diseases with inflammatory reaction. All COVID‐19 infected patients who were included in the infection group were positive for virus nucleic acid in nasopharyngeal swabs by real‐time fluorescence polymerase chain reaction (PCR), and those served as the noninfected group were negative. This study was approved by Ethics Committee of the First Hospital of Hunan University of Chinese Medicine, with an informed consent of patients and their families.
2.2. Laboratory analysis
Serum level of MMP3 was measured via latex‐enhanced immunoturbidimetry with a human MMP3 determination kit (Shanghai Huachen Biological Reagent Company), using the Cobas8000 automatic biochemical analyzer (Roche Company in Germany). The novel coronavirus nucleic acid extraction reagent (magnetic bead method; Jiangsu Master Biotechnology Co, Ltd, China) and novel coronavirus ORF1ab/N gene dual nucleic acid detection kit (fluorescence PCR method; Shanghai Huirui Biotechnology Co, Ltd, China) were used to viral nucleic acid test with the aid of the automatic nucleic acid extraction instrument (Jiangsu Master Biotechnology Co, Ltd, China). Interleukin 1β (IL‐1β) and IL‐6 test kits were purchased from Human Diagnostic Products Co, Ltd (Beijing). All tests were conducted by authorized, skilled laboratory personnel in accordance with the manufacturer's specifications and instructions.
2.3. Statistical analysis
Data were analyzed using SPSS (version 25.0) software. Normality tests were conducted for measurement data, where normal data were expressed as the mean and standard deviation, while t test was used for comparison between two groups. Data that did not conform to normality were expressed as the median and interquartile, and the Wilcoxon rank sum test was used to compare the differences in serum MMP3 levels between the two groups. Spearman's correlation analysis was used to explore the correlation between MMP3 and IL‐1β, and IL‐6. Statistical significance was set at P < .05.
3. RESULTS
3.1. Comparison of serum MMP3 between infected group and noninfected group
Normality tests indicated that data from both the novel coronavirus pneumonia infected group and the noninfected group were not normal. Therefore, the Wilcoxon rank sum test was used to compare the two groups. The MMP3 serum levels in the infected and noninfected groups are shown (Table 1). MMP3 concentrations were 44.44 (23.46 ~ 72.12) ng/mL in the infected group and 32.42 (28.16 ~ 41.21) ng/mL in the noninfected group. A positive correlation was found between infected and noninfected groups (Z = −2.799, P = .005 < .05). The distribution of MMP3 and the statistical significance between the two groups were plotted using a quartile box (Figure 1A).
Table 1.
Comparison of serum matrix metalloproteinase 3 concentration between the two groups (ng/mL)
| Group | N | Minimum | Maximum | M (P 25 ~ P 75) |
|---|---|---|---|---|
| Infected | 62 | 0.00 | 219.58 | 44.44 (23.46 ~ 72.12) |
| Noninfected | 131 | 9.84 | 72.33 | 32.42 (28.16 ~ 41.21) |
| Healthy | 67 | 9.84 | 64.59 | 29.68 (24.23 ~ 35.16) |
| Hospitalized | 64 | 21.04 | 72.33 | 37.08 (31.05 ~ 47.34) |
Figure 1.
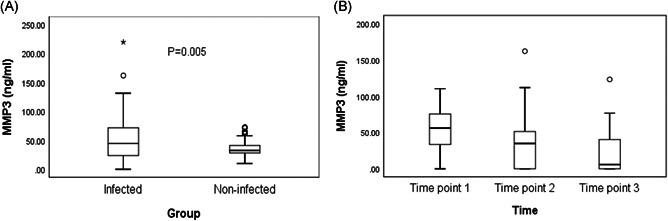
A, Comparison of serum concentration of MMP3 between the COVID‐19 infected group and noninfected group. Box above with the median as a line shows the distance between the quartiles, and the maximum and minimum values of MMP3 were presented by the whiskers. Outliers are displayed as separate points. P values show that there was a significant difference between the groups. B, Changes of serum MMP3 concentration in novel coronavirus patients during hospitalization. The interval of each test was 5 days, and a total of three times were tested. Box above with the median as a line shows the distance between the quartiles, and the maximum and minimum values of MMP3 were presented by the whiskers. Outliers are displayed as separate points. COVID‐19, coronavirus disease 2019; MMP, matrix metalloproteinase
3.2. Comparison of serum MMP3 among healthy people, infected group, and hospitalized patients
There was a significant difference in serum MMP3 between healthy people and COVID‐19 infected patients (Z = −3.414, P = .001 < .05). And a positive correlation was found between healthy people and hospitalized patients (Z = −4.644, P = .000 < .05). The distribution of MMP‐3 and the statistical significance were plotted using a quartile box (Figure 2A,B). And the MMP3 serum levels in healthy people and hospitalized patients are shown in Table 1.
Figure 2.
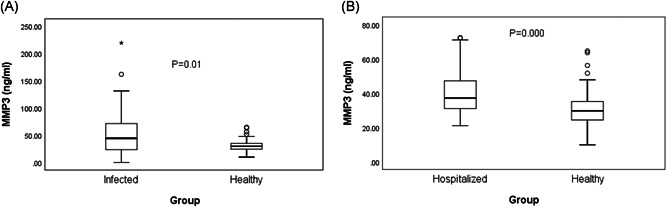
A, Comparison of serum concentration of MMP3 between the COVID‐19 infected group and healthy people. P values show that there was a significant difference between the groups. B, Comparison of serum concentration of MMP3 between the healthy people and noninfected hospitalized people. P values show that there was a significant difference between the groups. COVID‐19, coronavirus disease 2019; MMP, matrix metalloproteinase
3.3. Correlation between serum MMP3 and other inflammatory factors
Of the 62 patients with COVID‐19 infection, 31 were tested for MMP3, IL‐1β, and IL‐6 at the same time and their correlation was analyzed. Spearman's correlation analysis showed correlations between MMP‐3 and IL‐1β (r = .681, P = .000 < .05; Figure 3A), and IL‐6 (r = .529, P = .002 < .05; Figure 3B).
Figure 3.

A, Correlation between MMP3 and IL‐1β. B, Correlation between MMP3 and IL‐6. IL, interleukin; MMP, matrix metalloproteinase
3.4. Changes of serum MMP3 concentration in novel coronavirus patients during hospitalization
To detect serum MMP3 concentrations, sera from 20 patients were collected three times approximately every 5 days according to the duration of hospitalization. All were cured and discharged from hospital. Medians and interquartile ranges were used to describe these three serum MMP3 levels, the first time point being 55.98 (30.80 ~ 75.97) ng/mL, the second 34.84 (0.00 ~ 51.84) ng/mL, and the third 5.71 (0.00 ~ 40.46) ng/mL. The three serum MMP3 concentration levels showed a decreasing trend. The distribution of MMP‐3 serum concentrations over the three detection times is shown (Figure 1B).
4. DISCUSSION
Several reports have indicated that MMP3 may play an important role in the process of lung pathology, including ALI, ARDS, and pulmonary fibrosis. Studies conducted on animal models have found that the lungs of MMP3 gene‐deficient mice can be protected by inflammatory stimulation. 5 , 11 , 12 However, a study by Yamashita 13 indicated that MMP3 deficiency helps maintain the function of pulmonary surfactant to some extent and thereby protects the lungs from injury caused by physiological changes. This suggests that MMP3 plays a regulatory role in lung injury and repair. MMP3 is mainly secreted by fibroblasts and endothelial cells where inflammatory cells and cytokines can stimulate MMP3 secretion. It has been found that the nonmatrix substrates of MMP3 are proinflammatory cytokines such as IL‐1β and tumor necrosis factor α (TNF‐α). Furthermore, MMP3 also activates other MMPs, including pro‐MMP‐1, 3, 7, 8, 9, and 13. 6 , 14 , 15 While, the proteolysis function of MMP3 removes the adhesion sites between cells and the matrix, contributing to intracellular migration, MMP3 also plays a vital role in intercellular communication by regulating the activity of cytokines and chemokines, thus affecting and reflecting the progress of disease to a certain extent. 14 , 15
The current study compared the serum MMP3 concentrations of the novel coronavirus pneumonia infected group with those of the noninfected group. Our results indicated that MMP3 may be utilized to monitor the state of COVID‐19 patients. Some studies have identified the main pathological characteristics of COVID‐19 patients as pulmonary inflammation and lung injury, in addition to which many severe cases may also develop into SARS. 16 , 17 Current literature on COVID‐19 highlights the role of inflammation and immune responses in COVID‐19, which, together with cytokine storm and proinflammatory factors like IL‐1β and TNF‐α, undoubtedly contribute to the severity of disease. 18 , 19 Our study also shows that there is a positive correlation between MMP3 and inflammatory factors such as IL‐1β and IL‐6. It is important to consider the biological response of organisms to the SARS‐CoV‐2 infection from the point of view of protease and immune defense.
Hoffmann et al 20 found that novel coronavirus (SARS‐CoV‐2), enters the host cells in a manner similar to that of SARS‐CoV, relying on ACE2 and serine protease, TMPRSS2. This complex process via which the virus infects cells, involves a variety of proteases, and thus it is important to investigate antiviral intervention through correlative proteases. A recent study by Phillips et al 21 explored the role of various proteases in coronavirus infection and reported that zinc metalloproteases, such as MMP, may be potential contributors to coronavirus fusion. Therefore, it may be inferred that MMP3 is potentially associated with SARS‐CoV‐2 infection of host cells, via processes such as cell fusion. However, a literature review did not reveal any data on the association between MMP3 and coronavirus infection. In conclusion, future studies may lead to further progress in determining the relationship between MMP‐3 and SARS‐CoV‐2 infection.
This study has some limitations. First of all, due to a small number of patients, this paper cannot provide a comprehensive review of the correlation between MMP3 levels and disease severity. As COVID‐19, which is caused by a novel coronavirus virus, is a challenge for people all over the word, timely and effective measures can prevent the disease from causing a pandemic. Another potential problem is that, conditionally, the study did not take other diseases associated with COVID‐19 patients into account. And due to the limitations of the conditions, we did not make a statistical analysis of the detection of MMP3 according to the severity of the disease. A further study with more focus on excluding the influence of other factors is therefore suggested. Additionally, the future study may also contain the experiments on animal or cell model to explore more details.
In conclusion, the objective of the current study was to determine whether MMP3 levels have a role to play in the diagnosis of COVID‐19 patients. The results show that the detection of MMP3 may be the same as other inflammatory factors, which may reflect the inflammatory reaction process of part of COVID‐19 infected patients. Detection of serum MMP3 levels may be useful in diagnosis of COVID‐19 patients, as indicated by the positive correlation found between novel coronavirus pneumonia infected patients and noninfected patients. Insights gained from this study may assist in the monitoring, diagnosis, and treatment of COVID‐19. Thus, further studies regarding the role of MMP3 in the pathology of COVID‐19 are deemed to be useful.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
SS and MS contributed to the article drafting, revising, and data analysis. GS, FY, and XX contributed to the conception and design. YH, ZZ, PZ, GY, HZ, and QL contributed to data collection and technical analysis. XX is the guarantor for this study.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the First Hospital of Hunan University of Chinese Medicine.
ACKNOWLEDGMENTS
We would like to thank Editage (http://www.editage.cn) for English language suggestions. This study was supported by the Novel Coronavirus Pneumonia Emergency Project of Hunan Provincial Science and Technology Department (2020SK3018) and the First‐class Discipline Open Fund Project of Hunan University of Chinese Medicine (2018YXJS02).
Shi S, Su M, Shen G, et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID‐19. J Med Virol. 2021;93:528–532. 10.1002/jmv.26235
Contributor Information
Shengjie Shi, Email: xxiaobing888@163.com.
Xiaobing Xie, Email: xxiaobing888@163.com.
REFERENCES
- 1. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—an update on the status. Mil Med Res. 2020;7(1):11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yi F, Chen JL, Su M, et al. Clinical value of differential diagnosis of matrix metalloproteinase‐3 in benign and malignant hydrothorax and ascites. Chin J Lab Med. 2019;09:776‐781. [Google Scholar]
- 5. Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840(8):2571‐2580. 10.1016/j.bbagen.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 6. McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13(5):534‐540. 10.1016/s0955-0674(00)00248-9 [DOI] [PubMed] [Google Scholar]
- 7. Lerner A, Neidhöfer S, Reuter S, Matthias T. MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(4):550‐562. 10.1016/j.berh.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 8. Hattori Y, Kida D, Kaneko A. Normal serum matrix metalloproteinase‐3 levels can be used to predict clinical remission and normal physical function in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38(1):181‐187. 10.1007/s10067-017-3829-9 [DOI] [PubMed] [Google Scholar]
- 9. Davey A, McAuley DF, O'Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J. 2011;38(4):959‐970. 10.1183/09031936.00032111 [DOI] [PubMed] [Google Scholar]
- 10.General Office of National Health Commission of the People's Republic of China, Office of National Administration of Traditional Chinese Medicine. Diagnosis and treatment of corona virus disease‐19 (7th trial edition). https://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml
- 11. Nerusu KC, Warner RL, Bhagavathula N, McClintock SD, Johnson KJ, Varani J. Matrix metalloproteinase‐3 (stromelysin‐1) in acute inflammatory tissue injury. Exp Mol Pathol. 2007;83(2):169‐176. 10.1016/j.yexmp.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warner RL, Beltran L, Younkin EM, et al. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol. 2001;24(5):537‐544. 10.1165/ajrcmb.24.5.4160 [DOI] [PubMed] [Google Scholar]
- 13. Yamashita CM, Cybulskie C, Milos S, Zuo YY, McCaig LA, Veldhuizen RA. The effect of matrix metalloproteinase‐3 deficiency on pulmonary surfactant in a mouse model of acute lung injury. Can J Physiol Pharmacol. 2016;94(6):682‐685. 10.1139/cjpp-2015-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warner RL, Bhagavathula N, Nerusu KC, et al. Matrix metalloproteinases in acute inflammation: induction of MMP‐3 and MMP‐9 in fibroblasts and epithelial cells following exposure to pro‐inflammatory mediators in vitro. Exp Mol Pathol. 2004;76(3):189‐195. 10.1016/j.yexmp.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 15. Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840(8):2571‐2580. 10.1016/j.bbagen.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 16. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Yu L, Tang L, et al. A promising anti‐cytokine‐storm targeted therapy for COVID‐19: the artificial‐liver blood‐purification system. Engineering. 2020. 10.1016/j.eng.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:137244‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phillips JM, Gallagher T, Weiss SR. Neurovirulent murine coronavirus JHM.SD uses cellular zinc metalloproteases for virus entry and cell‐cell fusion. J Virol. 2017;91(8):e01564‐16. 10.1128/JVI.01564-16 [DOI] [PMC free article] [PubMed] [Google Scholar]


