Abstract
Cutaneous manifestations of COVID‐19 disease have not yet been fully described. To describe cutaneous manifestations of COVID‐19 disease in hospitalized patients. We examined the cutaneous manifestations of 210 hospitalized patients. Cutaneous findings were observed during COVID‐19 infection in 52 of the patients. Lesions may be classified as erythematous scaly rash (32.7%), maculopapular rash (23%), urticarial lesions (13.5%), petechial purpuric rash (7.7%), necrosis (7.7%), enanthema and apthous stomatitis (5.8%), vesicular rash (5.8%), pernio (1.9%), and pruritus (1.9%). Cutaneous manifestations were observed statistically significantly more in certain age groups: patients of 55 to 64 and 65 to 74 years of age complained of more cutaneous manifestations than the other age groups. As for gender, there was no significant difference between male and female patients in terms of cutaneus findings. The relationship between comorbidity and dermatological finding status was statistically significant. The relationship increases linearly according to the comorbidities. According to the statistical results, the patients who were hospitalized in the intensive care unit had a higher risk of having cutaneous findings due to COVID‐19 infection. With this study, we may highlight the importance of overlooked dermatological findings in patients that are hospitalized.
Keywords: coronavirus, COVID‐19, cutaneous manifestation, dermatological findings, skin
1. INTRODUCTION
In December 2019, first cases of pneumonia with unknown cause were reported in Wuhan, China. The pathogen, called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was isolated from lower respiratory tract of infected patients. The resulting disease was termed as COVID‐19 (Coronavirus Disease 2019). SARS‐CoV‐2 has rapidly spread throughout China and across the World, until reaching the level of a pandemic disease. 1 , 2 , 3 , 4
The SARS‐CoV‐2 infection mainly presents flu‐like symptoms such as fever, cough, asthenia and can affect different organ systems, probably including the skin. There are few descriptions of the cutaneous manifestations of COVID‐19. 5 Hydroxychloroquine (HCQ), Azitromycin (AZM), and oseltamivir are used in the standard treatment regime for COVID‐19 infection. Favipravir and tocilizumab are added to the regimen when necessary. 6 , 7 , 8 , 9 , 10
After our hospital was declared a pandemic hospital, private policlinics were opened. Between 10 March 2020 and 3 May 2020, 54,373 patients applied to outpatient clinics of Istanbul University‐Cerrahpasa, Cerrahpaşa Medical Faculty Hospital and 12 452 of these patients were tested for suspicion of COVID‐19. 1470 patients' tests were positive and 862 of them were hospitalized. 122 of these hospitalized patients needed intensive care.
We examined the cutaneous manifestations of 210 patients hospitalized in Cerrahpasa Medical Faculty, Istanbul, Turkey. During this pandemic, several case reports, and some articles were published about cutaneous manifestations of COVID‐19 infection. Cutaneous manifestations are important in the diagnosis of various viral infectious diseases. With this study, we may highlight the importance of overlooked dermatological findings in patients that are hospitalized.
2. MATERIALS AND METHODS
This study is a prospective observational study that evaluated 210 patients who were diagnosed as COVID‐19 and hospitalized in Istanbul University‐Cerrahpasa, Cerrahpasa Medical Faculty during April 2020. 47 of 210 patients were in the intensive care unit (ICU). We visited directly, got information about their medical history and took clinical images. The diagnosis of the patients was made according to computed tomography (CT) or reverse trancription‐polymerase chain reaction (RT‐PCR) results. RT‐PCR test of nasophrygeal or throat swabs is the gold standard for the diagnosis of COVID‐19; however, it is time consuming and may yield false‐negative results. 11 Although the RT‐PCR was negative, patients were hospitalized when the CT findings were positive. The typical imaging characteristics of patients with COVID‐19 are single or multiple ground‐glass opacity with subpleural distribution, crazy paving, and diffuse consolidation with ground‐glass opacity. 12 RT‐PCR and CT were performed to all patients and findings were recorded.
Clinicopathologic confirmation of the patients could not be made due to the pandemic conditions.
All patients gave their informed consent to participate and an explicit consent to use their pictures in publications.
Numerical and percentage represention was obtained by taking the frequency tables of the data. Comparison of cutaneus manifestations according to variables of hospitalization (ward vs intensive care unit), age, gender, RT‐PCR result was performed using chi‐square independence test. The difference in cutaneus findings by age groups was performed using chi‐square conformity analysis.
The present study was approved by the Clinical Research Ethics Committee of Istanbul University‐Cerrahpasa, Cerrahpasa Medical Faculty (approval number: 39122051‐604.01.01‐549 953; dated 22 April 2020).
3. RESULTS
Forty‐seven of 210 patients were in the intensive care unit (ICU). 58.6% (123) of them were male and 41.4% (87) were female. The average age of men were 57.44 ± 17.259, and the average age of women were 58.80 ± 15.918. While 41.9% (88) of the patients' SARS‐COV2‐PCR tests were negative, 58.1% (122) were positive. Again, 3.8% (8) negative CT findings were obtained, while 96.2% (202) had positive CT findings.
Seventy‐six of 210 (%36.1) patients had cutaneous findings. 52 of 210 patients (24%) had cutaneous findings that occured during COVID‐19 infection while in 24 of the patients had previously. While most of the previous findings were plantar hyperkeratosis, three of them were psoriatic plaques on the knee and elbow.
Clinical patterns in this 52 patients that occured during COVID‐19 infection:
Erythematous scaly rash 32.7% of cases (17). Most of the lesions were seen on hands (Figure 1). These erythmatous rashes were generally thought to be due to washing hands and using disinfectants.
Maculopapular rash 23% of cases (12). Most of the lesions were seen on the trunk, one was seen on the extremities and one was seen only on the upper part of the trunk. One of the lesion on the trunk was similar to pityriasis rosea (Figure 2).
Urticarial lesions 13.5% of cases (7). Mostly distributed in the whole body (Figure 3).
Petechial purpuric rash 7.7% of cases (4). While most of them were on the distal extremities (Figure 4) and petechial, one patient had generalized and petechial purpuric eruption. Platelet levels and coagulation tests of the patients were normal.
Necrosis 7.7% of cases (4). Necrosis was seen in one patient on the maxillary region (Figure 5), while most of them were in the compression areas such as sacrum (Figure 6).
Enanthema and apthous stomatitis 5.8% of cases (3). Rash and erythema were seen in the oral mucosa. In one patient, both enanthema and apthous stomatitis were seen together, while the other patient had an apthous lesion only one side of the tongue (Figure 7).
Vesicular rash 5.8% of cases (3). Two of them were unilateral and monomorphic on the upper part of the trunk (Figure 8).
Pernio 1.9% of cases (1). Cyanosis was seen in the acral areas in one patient (Figures 9 and 10).
Pruritus 1.9% of cases (1). The symptom was thought to be related to vancomycin, but clinicopathological confirmation could not be made.
FIGURE 1.
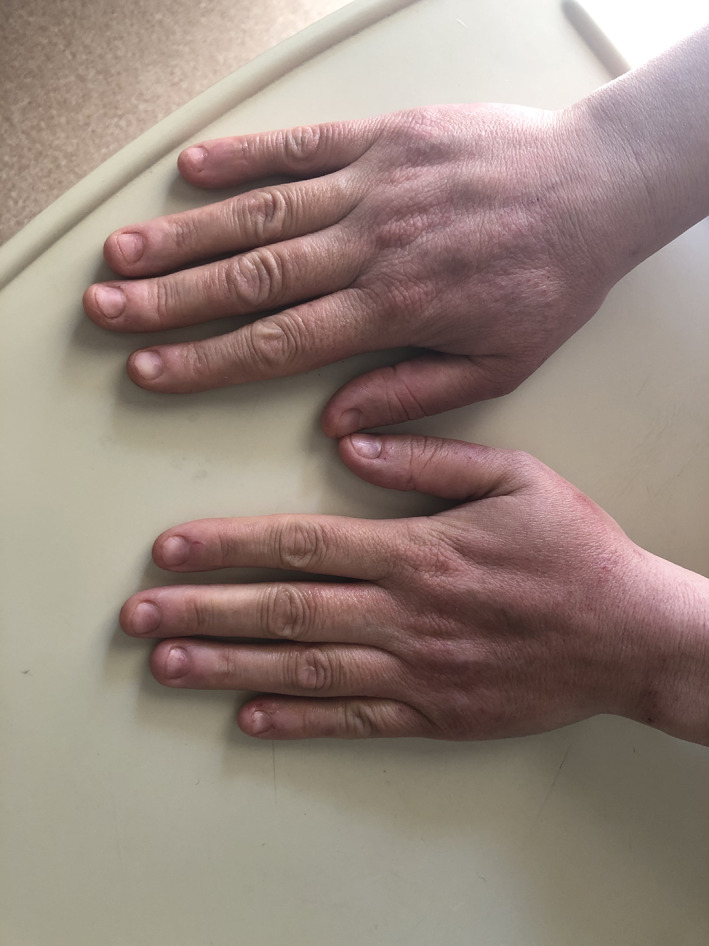
Irritant contact dermatitis on the hand
FIGURE 2.
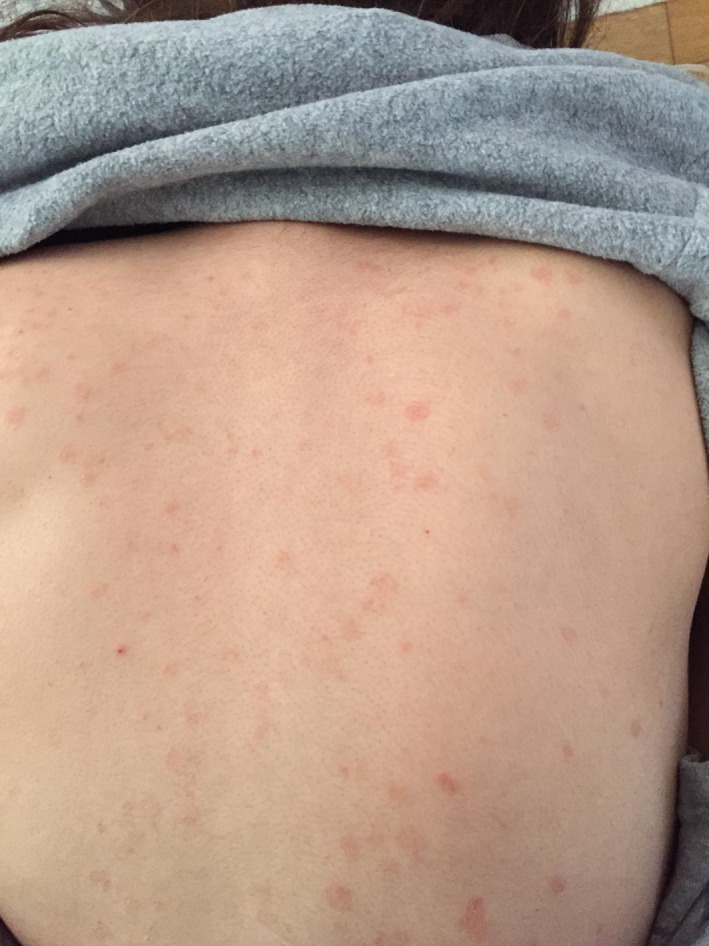
Macular rash on the trunk
FIGURE 3.
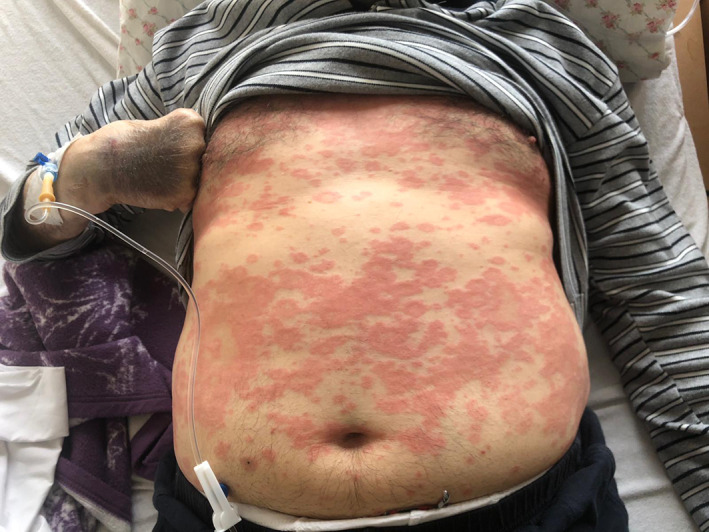
Urticarial lesions
FIGURE 4.
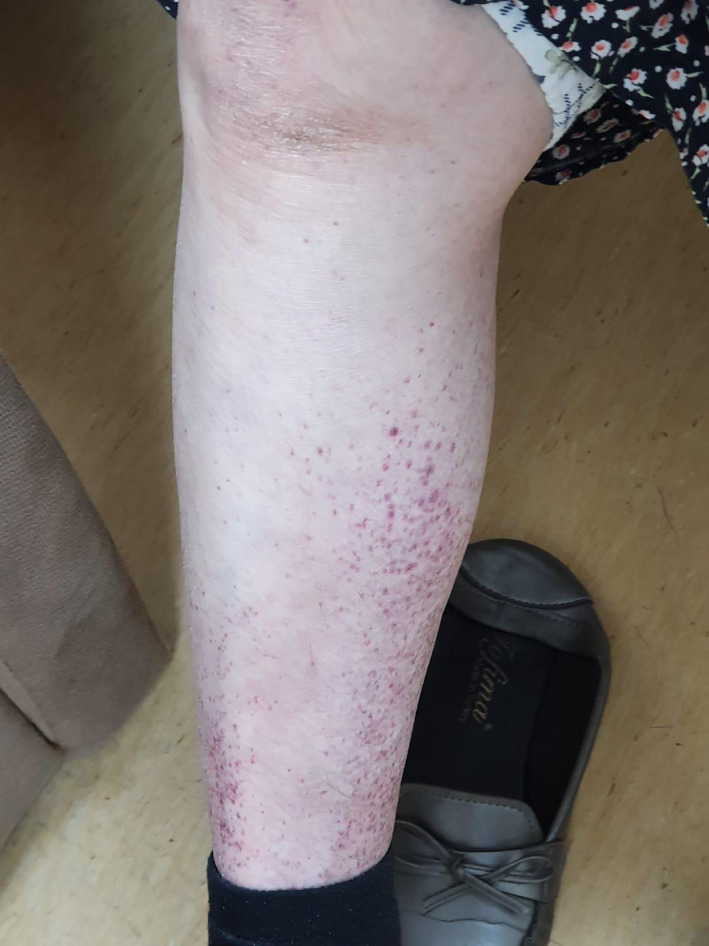
Petechial rash on the distal extremity
FIGURE 5.
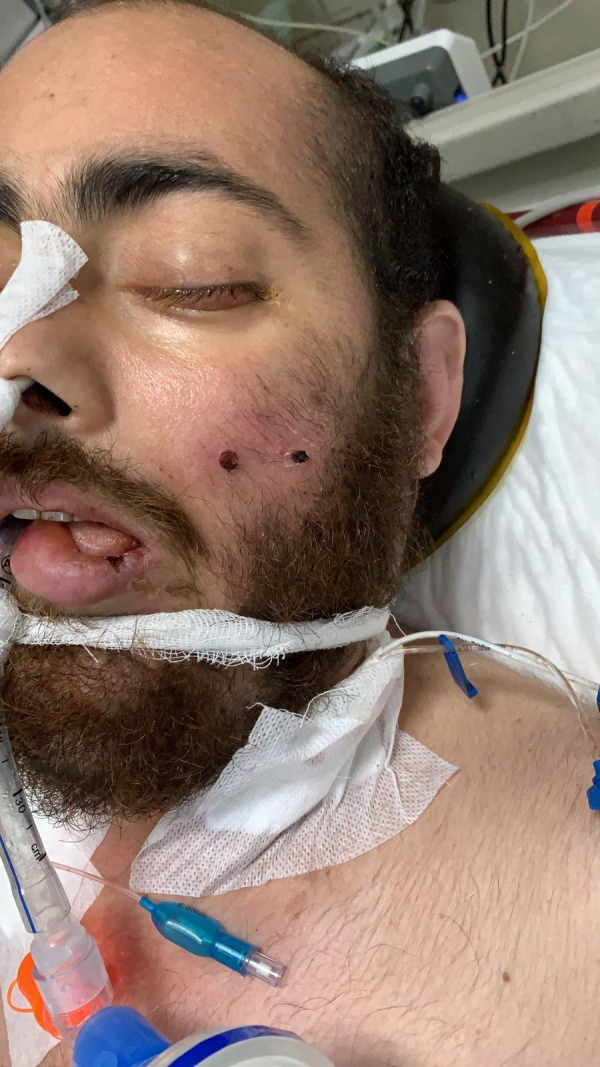
Necrotic lesion on the face
FIGURE 6.
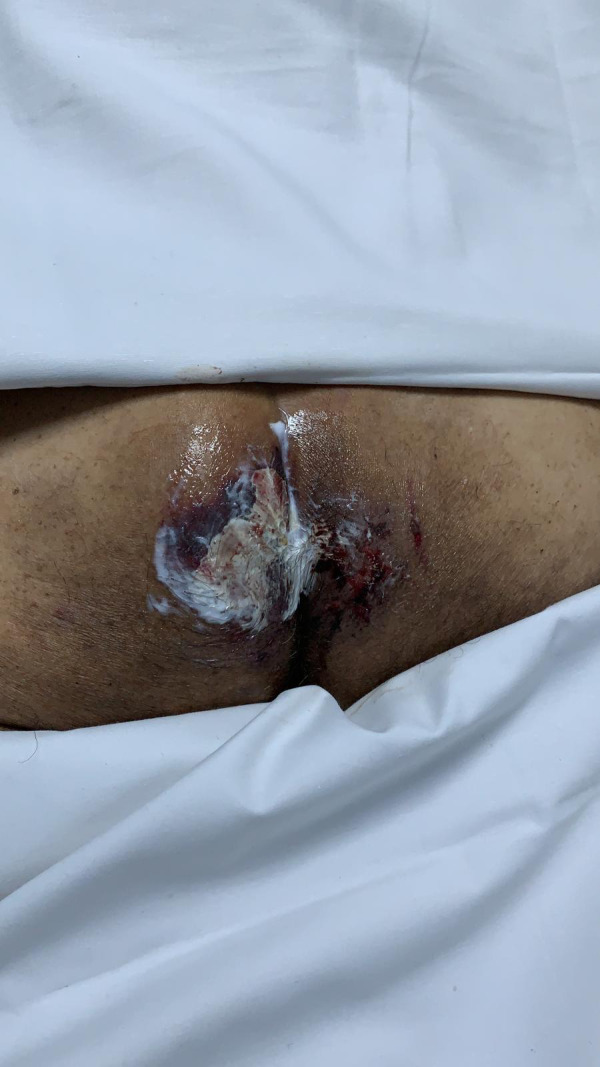
Necrotic lesion on the sacrum
FIGURE 7.
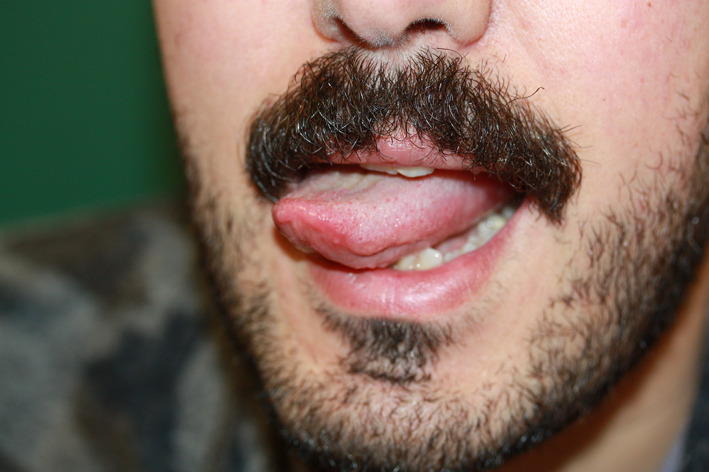
Aphtous stomatitis on the one side of the tongue
FIGURE 8.
Vesicular rash partially cured in thoracic region
FIGURE 9.
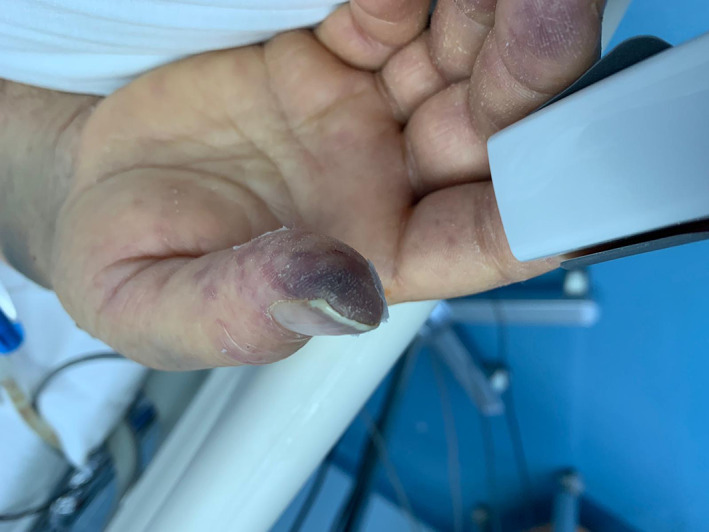
Cyanosis in the acral areas (pernio)
FIGURE 10.
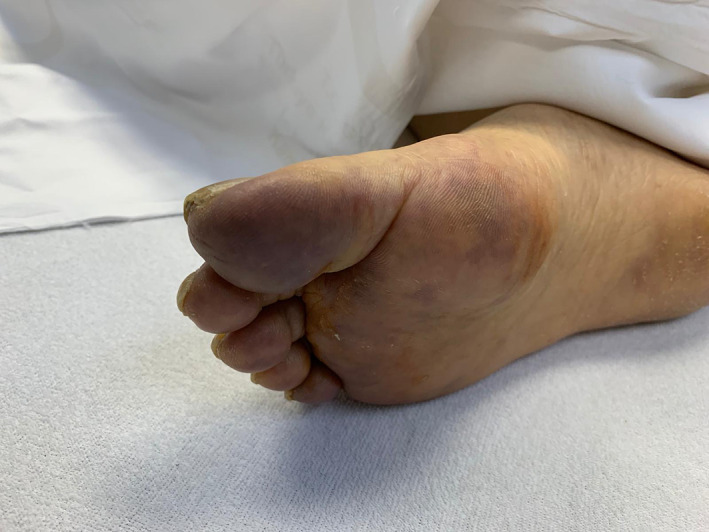
Cyanosis in the acral areas (pernio)
The relationship between age and cutaneous findings was examined by chi‐square independence test and a statistical relationship was obtained between them (χ2 = 25 538; SD = 6; P < .001). Accordingly, cutaneous findings were obtained more often in some age groups and this was statistically significant (P < .05) (Table 1).
TABLE 1.
Age group and cutaneous findings
| Cutaneous findings | Total | |||
|---|---|---|---|---|
| None | Exist | |||
| Age group | 18‐24 | 5 | 0 | 5 |
| 25‐34 | 15 | 3 | 18 | |
| 35‐44 | 20 | 6 | 26 | |
| 45‐54 | 28 | 6 | 34 | |
| 55‐64 | 31 | 19 | 50 | |
| 65‐74 | 33 | 10 | 43 | |
| 75‐84 | 22 | 5 | 27 | |
| 85++ | 4 | 3 | 7 | |
| Total | 158 | 52 | 210 | |
When cutaneous findings were analyzed according to the gender, 26.8% (33) of men and 21.8% (19) of women had dermatological findings. The relationship between gender and cutaneous findings was examined by chi‐square independence test and no statistical relationship was obtained between them (χ2 = 0.681; SD = 1; P > .05) (Table 2).
TABLE 2.
Gender and cutaneous findings
| Cutaneous findings | Total | |||
|---|---|---|---|---|
| None | Exist | |||
| Gender | Male | 90 | 33 | 123 |
| Female | 68 | 19 | 87 | |
| Total | 158 | 52 | 210 | |
The relationship between PCR positivity and cutaneous findings was examined by chi‐square independence test and no statistical relationship was obtained between them (χ2 = 1.990; SD = 1; P > .05) (Table 3).
TABLE 3.
PCR result and cutaneous findings
| Cutaneous findings | Total | |||
|---|---|---|---|---|
| None | Exist | |||
| PCR | Negative | 70 | 18 | 88 |
| Positive | 88 | 34 | 122 | |
| Total | 158 | 52 | 210 | |
In our study, 41.9% (88) of the patients did not have comorbidity, 22.9% (48) had one and 35.2% (74) had two or more comorbidities. When dermatological findings are examined according to comorbidity; dermatological findings were found in 21.1% (11) of those without comorbidity, 32.9% (17) of those with one, and 46.1% (24) of those with two comorbidities. The relationship between comorbidity and dermatological finding status was examined by chi‐square independence test and a statistically significance was obtained between them (χ2 = 21.562; SD = 2; P < .001). The relationship increases linearly according to the comorbidities (Table 4).
TABLE 4.
Comorbidities and cutaneous findings
| Cutaneous findings | Total (%) | |||
|---|---|---|---|---|
| None (%) | Exist (%) | |||
| Com | None | 53.7 | 21.1 | 41.9 |
| One | 17.2 | 32.9 | 22.9 | |
| Two + more | 29.1 | 46.1 | 35.2 | |
| Total | 100.0 | 100.0 | 100.0 | |
When the dermatological findings of the patients hospitalized in the ICU and in the ward are examined; cutaneous findings were found in 34 of 163 patients hospitalized in the service and 18 of 47 patients in the ICU.
The relationship between cutaneous findings and being in the ICU or being in the ward was examined by chi‐square independence test and a statistically significance was obtained between them (χ2 = 5.955; SD = 1; P < .05). It was determined that the patients who were hospitalized in the ICU had a higher risk of having cutaneous findings due to COVID‐19 infection (Table 5).
TABLE 5.
Differences in the appearance of cutaneous findings of patients in the wards and intensive care unit (ICU)
| Cutaneous findings | Total | ||
|---|---|---|---|
| None | Exist | ||
| In the wards | 129 | 34 | 163 |
| In ICU | 29 | 18 | 47 |
| Total | 158 | 52 | 210 |
4. DISCUSSION
In our study, we evaluated the cutaneous manifestations of the hospitalized patients due to the COVID‐19 pandemie. In our study, 24% of the patients who were hospitalized showed cutaneous findings during COVID‐19 infection. Recalcati et al 13 reported that 20.4% of the 88 patients diagnosed with COVID‐19 had cutaneous findings such as erythematous rash, widespread urticaria, and chickenpox‐like vesicles. Also a Spanish medical ward collected nationwide data from 375 patients and classified lesions as acral areas of erythema with vesicles or pustules (pseudo‐chilblain), other vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis. 14 Of the 47 patients who were hospitalized at the ICU 38.2% (18) of them showed cutaneous findings in current study. The greater percentage of the patients at the ICU may be due to the severe infection. Five studies reported on the possible association between COVID‐19 and skin lesion severity in 23 patients. In 21 patients (91.3%), the severity of cutaneous lesions was unlikely to be or was not correlated with COVID‐19 severity. Contrarily, in two separate reports by Mahe and Estébanez, COVID‐19 and skin lesion severity were linked in two patients (8.7%). 15 The patients who had comorbidities showed a greater percentage of cutaneous manifestations than those without comorbidities. According to our study, there was no significant difference in the frequency of dermatological findings in men and women. In a literature review that analyzed 16 articles, showed that of the 50 patients with determined gender, 27 (54%) were males and 23 (46%) were females. 16 In another literature review that analyzed 18 articles, males accounted for 38.9% of reported cases, females accounted for 27.8% of cases. 17 The result of our study was also compatible with these articles.
The most commonly observed skin eruption in COVID‐19 patients was erythematous scaly rash in our study. The increased frequency of hand‐washing and the use of the disinfectants increase the risk of irritant contact dermatitis. 18 Maculopapular skin rash and urticarial rash were the second and third most commonly seen cutaneous manifestations. Maculopapular rash was most commonly seen on the trunk; urticarial lesions were generalized. Maculopapular lesions and urticaria may occur due to COVID‐19 disease itself or the antiviral drugs used in the treatment. 15 The increased frequency of maculopapular rash and urticaria in our study group is in alignment with the previously reported literature. 15 , 16 , 19 Petechial and purpuric lesions and necrosis were also observed in our study group. A case series from China reported vasculopathic skin lesions ranged from acral cyanosis to dry gangrenes in seven patients. 20 Unlike the previously reported publishings, neither “chickenpox‐like vesicles” nor chillblain‐like symptoms were not observed in our study. There was only one patient with the diagnosis of pernio.
The limitation of our study was that we just evaluated the hospitalized patients, and we could not perform clinicopathological confirmation. Our study is the first prospective study that evaluates the patients' cutaneous findings at the COVID‐19 wards or the COVID‐19 ICU in Turkey. In this regard, our study may give an opinion to the dermatologists about moderate/severe COVID‐19 infections' cutaneous manifestations.
Askin O, Altunkalem RN, Altinisik DD, Uzuncakmak TK, Tursen U, Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID‐19. Dermatologic Therapy. 2020;33:e13896. 10.1111/dth.13896
REFERENCES
- 1. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng OT, Marimuthu K, Chia PY, et al. SARS‐CoV‐2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476‐1478. 10.1056/NEJMc2003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for Global Health governance. JAMA. 2020;323:709. 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 4. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu D, Wu T, Liu Q, et al. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020;94:44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savarino A, di Trani L, Donatelli I, et al. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67‐69. 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao B, Wang Y, Wen D, et al. Trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C et al. Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. bioRxiv. 2020;17(39). 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 11. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kay F, Abbara S. The many faces of COVID‐19: spectrum of imaging manifestations. Radiol Cardiothor Imag. 2020;2(1):e200037. 10.1148/ryct.2020200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212‐e213. 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 14. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;180(1):71‐77. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID‐19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75‐81. 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang K, Wang Y, Zhang H, et al. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID‐19): a brief review. Dermatol Ther. 2020;e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suchonwanit P, Leerunyakul K, Kositkuljorn C. Cutaneous manifestations in COVID‐19: lessons learned from current evidence. J Am Acad Dermatol. 2020;83(1):e57‐e60. 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID‐19 prevention can cause hand dermatitis: management tips. Cureus. 2020;2(12):4, e7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tammaro A, Adebanjo GAR, Parisella FR, et al. Cutaneous manifestations in COVID‐19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34(7):e306‐e307. 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]


