Summary
COVID‐19 is associated with increased risk of venous thromboembolic events (VTE). However, there is significant heterogeneity in the thromboembolic phenotypes of COVID‐19 patients (deep vein thrombosis, pulmonary embolism/thrombosis). The latter might be partly attributed to the variation in VTE risk factors in COVID‐19 patients including: (i) patients’ characteristics; (ii) hospitalization conditions and interventions; and (iii) SARS‐CoV‐2‐specific factors (coagulopathy, endothelial injury/microthrombosis). Furthermore, there is methodological heterogeneity in relation to the assessment of VTE (indications for screening, diagnostic methodology, etc). Physicians should be aware of the increased VTE risk, strongly consider VTE screening, and use thromboprophylaxis in all hospitalized patients.
Keywords: SARS‐CoV‐2, pulmonary embolism, deep vein thrombosis, prevalence
Accumulating evidence suggests that severe coronavirus disease 2019 (COVID‐19) is associated with an increased venous thromboembolic risk. 1 , 2 It appears that SARS‐CoV‐2 in severe cases induces an excessive immune response associated with a cytokine storm leading in turn to coagulation disorders. 1 , 2 The latter can be observed at both local level with lung endothelial injury and microthothrombosis, as well as at systematic level with disseminated intravascular coagulopathy. 1 , 2
In light of the emerging evidence on the thromboembolic risk in COVID‐19, recent publications highlight two important issues: firstly the high rate of venous thromboembolic events (VTE) in COVID‐19 patients, and secondly the variety of the observed thromboembolic phenotypes. Indeed, 11 recent studies reported both the prevalence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in COVID‐19 patients with prevalence numbers ranging from 0% to as high as 54% (Table I). 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Importantly, there was no consistent relationship between the reported prevalence of DVT and PE. It should be mentioned that most studies have included mainly patients in the intensive care unit (ICU) who presumably had severe COVID‐19 (Table I). One study reported that the prevalence of VTE was significantly higher in ICU versus general‐ward patients (47% and 3% respectively). 6
Table I.
Main characteristics and findings of studies.
| Study | Setting | N | Age, years ± SD (range) | Males (%) | Prevalence of DM/CVD/PD (%) | Median (range) SOFA/PaO2/FiO2 | Antithrombotic treatment dosing | Prevalence of DVT/PE (%) | D‐dimer (μg/ml; median values) and predictive value (ratio and 95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Stoneham et al. 3 | General ward | 274 | VTE 67 ± 12 | VTE 67 | VTE 38/29/38 | NR | NR | 2/6 |
VTE vs. non‐VTE: 4·1 vs. 1·2 Adjusted OR for VTE: 1·4 (1·2,1·8) |
| Wright et al. 4 | ICU | 44 | 54 (19–86) | 64 | 41/NR/14 | 8 (7–10)/163 (127–235) | Prophylactic | 25/0 | 1·8 (0·9–4·1) |
| Thomas et al. 5 | ICU | 63 | 59 ± 13 | 69 | NR | NR | Prophylactic | 2/8 | 0·4 (0·1–3·6) |
| Middeldorp et al. 6 | General ward 62%; ICU 38% | 198 | 61 ± 14 | 66 | NR | NR | Mainly prophylactic | 13/7 |
VTE vs. non‐VTE: 2·6 vs. 1·0 Subhazard ratio for VTE: 1·4 (1·1,1·9) |
| Helms et al. 7 | ICU | 150 | 63 (53–71) | 81 | 20/48/14 | 8 (5–10)/125 (97–170) | Mainly prophylactic | 2/17 | 2·3 (1·2–20·0) |
| Lodigiani et al. 8 | General ward 84%; ICU 16% | 388 | 66 (55–85) | 68 | 23/33/9 | NR | Mixed doses | 2/3 | Rapid increase in D‐dimer in non‐survivors |
| Poissy et al. 9 | ICU | 107 | PE 57 (29–80) | PE 59 | NR |
PE 4 (0–4)/NR |
Prophylactic | 5/21 | Subhazard ratio for PE: 1·8 (1·0,3·2) |
| Tavazzi et al. 10 | ICU | 54 | VTE 68 ± 7 | NR | NR | NR | Prophylactic | 15/6 | NR |
| Llitjos et al. 11 | ICU | 26 | 68 (52–75) | 77 | NR | 3 (2–5)/87 (74–116) | Mainly therapeutic | 54/23 | 1·8 (1·1–2·9) |
| Beun et al. 12 | ICU | 75 | NR | NR | NR | NR | NR | 4/27 | NR |
| Klok et al. 13 | ICU | 184 | 64 ± 12 | 76 | NR | NR | Mainly prophylactic | 2/35 | NR |
CI, confidence intervals; CVD, cardiovascular disease; DM, diabetes mellitus; DVT, deep vein thrombosis; FiO2, fraction of inspired oxygen; ICU, intensive care unit; NR, not reported; OR, odds ratio; PaO2, arterial partial pressure of oxygen; PD, pulmonary disease; PE, pulmonary embolism; SD, standard deviation; SOFA, Sequential Organ Failure Assessment; VTE, venous thromboembolic events.
These data indicate that there is heterogeneity in the reported VTE risk — although recognized by all as increased — as well as in the thromboembolic phenotypes of COVID‐19 patients (isolated DVT, isolated pulmonary embolism/thrombosis, concurrent DVT and pulmonary embolism/thrombosis). It might be suggested that variation in several VTE risk factors in COVID‐19 patients accounts for this observed heterogeneity; risk factors are presented in Fig 1 and include: (i) characteristics of the patients including well‐established risk factors for VTE; (ii) hospitalization conditions and interventions; and (iii) SARS‐CoV‐2‐specific factors.
Figure 1.
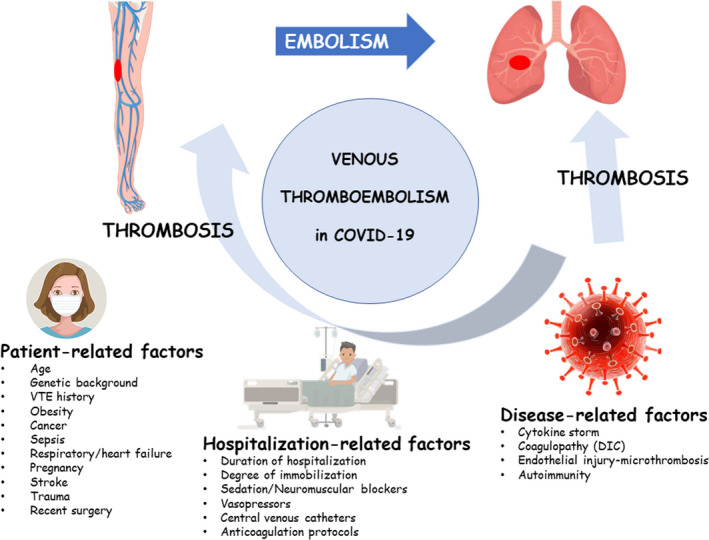
Factors increasing the risk of venous thromboembolism in COVID‐19. [Colour figure can be viewed at wileyonlinelibrary.com]
In addition to the above, a further important issue is the heterogeneity in the methodology used across studies to identify VTE in COVID‐19 patients. Indeed, factors that might play a role include: (i) indications for VTE screening; i.e. consecutive patients or selected ones upon clinical (respiratory or haemodynamic deterioration) or biochemical (increase in D‐dimer values) suspicion; and (ii) the diagnostic methodology applied, that is, ultrasonography or computed tomography pulmonary angiography or both, which is largely dependent on the available human and equipment resources.
Interestingly, even in studies that reported screening for DVT with leg compression ultrasonography in all their patients, there has been significant heterogeneity. Specifically, Ren et al. reported that among 48 critically ill COVID‐19 patients hospitalized in the ICU, 41 (85%) presented with lower‐extremity DVT, mainly in the pattern of isolated distal DVT. 14 On the contrary, in another study in 64 COVID‐19 patients hospitalized in the general ward, none was found with DVT. 15 The two studies included patients with a similar age (median 70 years) and gender distribution, yet the former study included patients admitted in ICU with a more severe disease and 7‐fold higher D‐dimer levels. 14 , 15 It should be mentioned that thromboprophylaxis was administered in both studies. 14 , 15
The role of D‐dimer assessment and of optimal thromboprophylaxis in COVID‐19 patients is of paramount importance. Some studies have shown that increased D‐dimer predict the development of VTE. 3 , 6 , 9 Thus, patients with increased D‐dimer values on admission or increasing D‐dimer values during their hospitalization should be candidates for VTE screening. Moreover, several societies now recommend the use of thromboprophylaxis in all hospitalized patients. 1 Although prophylactic dosing is generally recommended, some experts consider the use of intermediate dosing but relevant studies are lacking.
In summary, and in terms of clinical practice, physicians dealing with COVID‐19 patients should be aware that: (i) the risk of venous thromboembolism is high, yet with variable incidence of phenotypes (DVT and PE); (ii) all hospitalized patients require thromboprophylaxis, yet the optimal dosing is uncertain; and (iii) VTE screening should be strongly considered and influenced by clinical and biochemical characteristics (D‐dimer).
Author contributions
AK and KGK performed the research and drafted the manuscript. GSS and KS provided critical review and supervision.
References
- 1. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–7. 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID‐19. Intensive Care Med. 2020:1–4. 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoneham SM, Milne KM, Nuttal E, Frew GH, Sturrock BR, Sivaloganathan H, et al. Thrombotic risk in COVID‐19: a case series and case‐control study. Clin Med (Lond). 2020;20(4):e76–e81. 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID‐19 infection. J Am Coll Surg. 2020;231:193–203.e1. 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, et al. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–7. 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients in severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98. 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–6. 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 10. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli, F . Thrombotic events in SARS‐CoV‐2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;46(6):1121–3. 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18(7):1743–6. 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42(S1):19–20. 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;3848(20):148–50. 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren B, Yan F, Deng Z, Zhang S, Xiao L, Wu M, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID‐19 in Wuhan. Circulation. 2020;142:181–3. 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 15. Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020. 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]


