Abstract
Coronavirus disease 2019 (COVID‐19) is generally a relatively mild illness in children. An emerging disease entity coined as pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS) has been reported recently, but is very rare and only affects a very small minority of children. Here we describe the clinical presentations and outcomes of three teenagers with serologically‐confirmed SARS‐CoV‐2 infection admitted to a pediatric intensive care unit for PIMS‐TS. Although their initial presentations were very similar, their COVID‐19‐related disease varied in severity.
Keywords: coronavirus, COVID‐19, inflammatory, multisystem, SARS‐CoV‐2
Highlights
A novel condition called pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS) is emerging.
Here we describe three teenage cases of PIMS‐TS who were all confirmed with positive SARS‐CoV‐2 tests.
The pathogenesis of PIMS‐TS is still largely unknown but is likely immune‐mediated, similar to that of Kawasaki disease shock syndrome or macrophage activation syndrome.
1. BACKGROUND
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the cause of a rapidly spreading illness, coronavirus disease 2019 (COVID‐19). The virus has caused a devastating pandemic since its origins in Wuhan, Hubei province of China in December 2019, affecting more than six million people worldwide and with over 350 000 deaths, according to the World Health Organization (WHO) COVID‐19 Dashboard (accessed on 3 June 2020). 1 COVID‐19 has resulted in almost 40 000 deaths in the United Kingdom (UK) as of 6 June 2020. 2
Previously published studies have reported on the relatively benign impact of SARS‐CoV‐2 infections in children. 3 However, in April 2020, there was an emergence of a new multisystem inflammatory illness affecting children related to COVID‐19 coined as “pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2” (PIMS‐TS). 4 , 5 , 6 The majority of these patients required admission to pediatric intensive care units (PICU), necessitating input from various specialists including cardiologists, rheumatologists, immunologists, and infectious disease specialists. Patients with PIMS‐TS exhibit similarities to diseases such as Kawasaki disease (KD) and streptococcal and staphylococcal toxic shock syndromes (TSS). 7 , 8 , 9
2. REPORT OF THREE CASES WITH PIMS‐TS
We describe PIMS‐TS in three teenagers admitted to our PICU between April 2020 and May 2020, who required PICU admission, including their clinical presentations, investigations, and management (Table 1).
Table 1.
Characteristics of patients admitted for PIMS‐TS
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age, y | 16 | 17 | 13 |
| Sex | Female | Male | Male |
| Ethnicity | Asian Indian | Afro‐Caribbean | Afro‐Caribbean |
| Body mass index (percentile) | 22 kg/m2 (25‐50th) | 23.3 kg/m2 (75th‐91st) | 36.7 kg/m2 (>99.6th) |
| Clinical features | Fever, sore throat, conjunctivitis, cervical lymphadenopathy, maculopapular rash, diarrhea, vomiting, abdominal pain, confusion, dyspnea, hypotension, tachycardia | Fever, sore throat, conjunctivitis, cervical lymphadenopathy, cracked lips, diarrhea, vomiting, abdominal pain, hypotension, tachycardia | Fever, conjunctivitis, urticarial rash, diarrhea, vomiting, abdominal pain, headache, lethargy, dyspnea, hypotension, tachycardia |
| Duration of symptoms before admission | 8 d | 5 d | 6 d |
| Abnormal laboratory results | |||
| Inflammatory markers | CRP 403 mg/L, procalcitonin >75 ng/mL, ferritin 1342 μg/L, LBP 66 μg/mL, IL‐6 11.6 pg/mL | CRP 399 mg/L, procalcitonin 23.6 ng/mL, ferritin 5440 μg/L, LBP 94.5 μg/mL, IL‐6 471.5 pg/mL | CRP 328 mg/L, procalcitonin 4.3 ng/mL, ferritin 540 μg/L, LBP 63.3 μg/mL, IL‐6 137.5 pg/mL |
| Full blood count (x109/L) | WBC 23.1, neutrophil 21.3, lymphocyte 0.87, platelet 636, | WBC 27.7, neutrophil 24.71, lymphocyte 0.45, platelet 487 | WBC 38.1, neutrophil 36.2, lymphocyte 1.15, platelet 111 |
| Hematology | Hb 86 g/L, D‐dimer 12.96 μg/mL FEU, fibrinogen 4.8 g/L, INR 1.3, PT 17.5 s, APTT 35.4 s | D‐dimer 4.36 μg/mL FEU, fibrinogen 8.1 g/L, INR 1.3, PT 17.4 s, APTT 41.1 s | Hb 76 g/L, D‐dimer 9.41 μg/mL FEU, fibrinogen 8.1 g/L, INR 2.2, PT 28.8 s, APTT 68.6 s |
| Renal function and serum electrolytes | Creatinine 135 μmol/L, magnesium 0.66 mmol/L | Creatinine 99 μmol/L, sodium 127 mmol/L, phosphate 0.52 mmol/L | Creatinine 428 μmol/L, adjusted calcium 1.57 mmol/L, sodium 157 mmol/L |
| Liver function and other biochemistry | Albumin 18 g/L, LDH 440 iu/L, CK 406 iu/L | Albumin 22 g/L, ALT 80 iu/L | Albumin 21 g/L, ALT 69 iu/L, AST 73 iu/L, GGT 184 iu/L, Amylase 250 iu/L, LDH 374 iu/L |
| Cardiac markers | Troponin I 821.6 ng/L, NT‐ProBNP 13 222 ng/L | Troponin I 1766.5 ng/L, NT‐ProBNP 18 620 ng/L | Troponin I 2035.1 ng/L, NT‐ProBNP 22 841 ng/L |
| Hormonal studies | PTH 18.18 pmol/L, 25‐hydroxy vitamin D 19 nmol/L | Cortisol 1471 nmol/L | 25‐hydroxy vitamin D < 15 nmol/L |
| Imaging findings | CXR: bilateral basal and peripheral airspace shadowing, no cardiomegaly | CXR: cardiomegaly, retrocardiac and left lower lobe airspace opacification, left pleural effusion | CXR: right upper lobe collapse‐consolidation, no cardiomegaly |
| Echocardiography: mildly impaired left ventricular function, no coronary arteries dilation, small pericardial effusion. Incidental left pleural effusion | CT head: normal | Echocardiography: Normal function. Mild mitral regurgitation. Dilated RCA 4.6 mm (Z‐score +2.2) and LCA 4.9‐5.7 mm (Z‐score +2.2‐3.7). No pericardial effusion | |
| Echocardiography: minimal pericardial effusion, good function, RCA 4.9 mm ectasia (Z‐score +3) | |||
| Microbiological results | |||
| Bacterial | Blood culture no growth | Blood culture no growth | Blood culture no growth |
| No pyuria | No pyuria | Sterile pyuria (White cells >100 × 106/L) | |
| Blood meningococcal and pneumococcal PCR negative | Throat swab no growth Fecal culture no growth | Endotracheal secretion culture no growth | |
| ASOT twice <200 iu/mL (6 d apart) | ASOT < 200 iu/mL, 6 d later >400 iu/mL | Throat swab no growth | |
| Urine Legionella pneumophila and pneumococcal antigen negative | Fecal culture no growth | ||
| Blood meningococcal and pneumococcal PCR negative | |||
| Virology | Nasopharyngeal swab SARS‐CoV‐2 PCR positive | Nasopharyngeal swab SARS‐CoV‐2, adenovirus, enterovirus and parechovirus PCR negative | Endotracheal secretion and nasopharyngeal swab SARS‐CoV‐2, influenza, RSV, adenovirus, enterovirus, and parechovirus PCR negative |
| SARS‐CoV‐2 IgG positive (Day 9 of illness) | SARS‐CoV‐2 IgG positive (Day 5 of illness) | SARS‐CoV‐2 IgG positive (Day 7 of illness) | |
| EBV VCA IgM negative, IgG positive | Fecal rotavirus, norovirus and adenovirus antigen negative | Blood EBV DNA PCR 169 iu/mL | |
| Fecal echovirus, parechovirus and adenovirus PCR, rotavirus and norovirus antigen negative | Blood enterovirus PCR negative | ||
| Treatment | |||
| Highest respiratory support (duration) | Nasal prong oxygen 2 L/min (4 d) | HHFNC 25 L/min with FiO2 30% (1 d) | Mechanical ventilation (5 d) |
| Fluid bolus | 50 mL/kg | 20 mL/kg | 160 mL/kg |
| Inotropic support | Adrenaline infusion | … | Adrenaline, noradrenaline and vasopressin infusions, hydrocortisone |
| Antibiotics | Ceftriaxone, clindamycin | Ceftriaxone, clindamycin | Piperacillin‐tazobactam, ceftriaxone, clindamycin, meropenem |
| Anti‐inflammatory agents | … | IVIG 2 g/kg/day for 2 d | Methylprednisolone 2 mg/kg/day |
| Aspirin 30 mg/kg/day for 2 d | IVIG 2 g/kg/day for 2 d | ||
| Aspirin 30 mg/kg/day for 1 d | |||
| Antiplatelet | … | Aspirin 75 mg daily | Aspirin 75 mg daily |
| Length of PICU stay | 4 d | 3 d | 10 d |
| Length of hospital stay | 13 d | 13 d | 16 d |
Abbreviations: ALT, alanine transaminase; APTT, activated partial thromboplastin time; ASOT, antistreptolysin O titer; AST, aspartate transaminase; CK, creatine kinase; CRP, C‐reactive protein; CT, computed tomography; CXR, chest X‐ray; DNA, deoxyribonucleic acid; EBV, Ebstein‐Barr virus; FiO2, fraction of inspired oxygen; Hb, hemoglobin; HHFNC, humidified high flow nasal cannula; IgG, immunoglobulin G; IgM, Immunoglobulin M; IL‐6, interleukin‐6; INR, international normalized ratio; IVIG, intravenous immunoglobulin; LBP, lipopolysaccharide‐binding protein; LCA, left coronary artery; LDH, lactate dehydrogenase; NT‐ProBNP, NT‐proB‐type natriuretic peptide; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; PT, prothrombin time; PTH, parathyroid hormone; RCA, right coronary artery; RNA, ribonucleic acid; RSV, respiratory syncytial virus; VCA, viral capsid antigen; WBC, white blood cell.
All of these cases were adolescents and they were of Afro‐Caribbean and Asian ethnic backgrounds. Cases 1 and 2 did not have any co‐morbidities but Case 3 was morbidly obese. They had almost identical presentations with overlapping symptoms and laboratory parameters of KD and TSS. All three patients presented with fever, conjunctivitis, rash, gastrointestinal symptoms, and circulatory shock, requiring aggressive fluid therapy. None of the patients had contact history with COVID‐19 patients.
Only one (Case 1) of the three cases had tested positive for SARS‐CoV‐2 by polymerase chain reaction (PCR) testing on day 8 of illness. The other two cases had both tested negative on multiple samples that were taken around the time of their acute presentation. However, SARS‐CoV‐2 immunoglobulin G (IgG) was positive for all three cases. Case 1 tested positive for SARS‐CoV‐2 IgG on day 9 of illness, Case 2 on day 5 of illness, and Case 3 on day 7 of illness. The serology testing was performed using the DiaSorin (Saluggia VC, Italy) Liaison SARS‐CoV‐2 S1/S2 IgG assay, which detects antibodies specific to the SARS‐CoV‐2 spike (S) proteins, using magnetic beads coated with SARS‐CoV‐2 S antigens. The assay has a sensitivity of 90.4% between 5 and 15 days after initial infection, increasing to 97.4% post 15 days. In addition, Case 2 had seroconversion suggestive of acute Group A streptococcal infection, and Case 3 had low‐level Epstein‐Barr virus viremia.
All three cases had striking inflammatory markers results, severe neutrophilia, lymphopenia, and biochemical findings consistent with myocarditis. The trends of full blood count, coagulation profile, and biochemistry results are shown in Figure 1. In the early phase of their presentations, there was leucocytosis, neutrophilia, and lymphopenia which gradually improved over time. All three cases had low/normal platelet count in the beginning followed by a rising platelet trend in their course of the disease. Cases 1 and 3 developed anemia. There was also prolonged prothrombin time and activated partial thromboplastin time, high international normalised ratio, fibrinogen, D‐dimer, C‐reactive protein (CRP) and creatinine in the beginning but their values decreased as they recovered from their illness. In the active stage of their condition, the albumin level fell to as low as 18 g/L. Two patients had acute kidney injury (Cases 1 and 3), liver dysfunction (Cases 2 and 3), and coronary artery dilatation (Cases 2 and 3). Case 2 had borderline raised creatinine. Case 2's electrocardiogram showed ST depression in lead II, III, and AVF.
Figure 1.
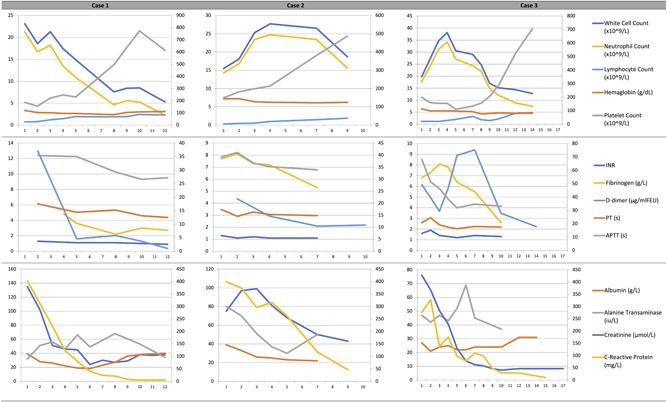
Line graph representation of full blood count, coagulation profile and biochemistry results for all three cases according to day of admission
Figure 2 shows the serial chest X‐ray (CXR) images of all three cases on admission. The two CXRs of Case 1 (done 1 day apart) showed bilateral basal and peripheral airspace shadowing but there was no cardiomegaly. Case 2's first CXR revealed only mild perihilar inflammatory changes without cardiomegaly but his second CXR progressed to show cardiomegaly, left pleural effusion, retrocardiac, and left lower lobe airspace opacification. Case 3's first CXR was normal but later developed right upper lobe collapse consolidation. There was no obvious cardiomegaly in the second CXR of Case 3.
Figure 2.
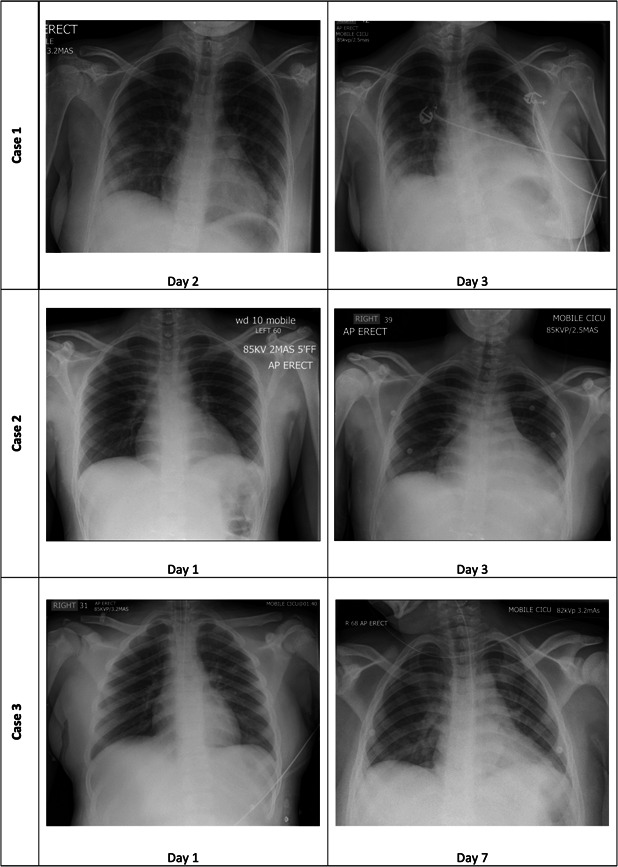
Chest radiographs of all three cases according to day of admission
All three cases required respiratory support, one was placed on invasive ventilation (Case 3) and two patients needed inotropic support (Cases 1 and 3). Cases 2 and 3 were given intravenous immunoglobulin (IVIG) infusions and Case 3 received corticosteroid therapy. All three cases recovered and they were given follow up appointments with the pediatric cardiologists upon discharge. A clinical audit on these patients has been set up within our institution to look at long term outcomes and multisystem sequelae of this new condition.
3. DISCUSSION
Children with SARS‐CoV infection were initially reported to be only mildly affected with very few admissions to PICU and very few deaths reported. 3 They can be asymptomatic or may present with fever, respiratory and/or gastrointestinal symptoms. 3
The emergence of PIMS‐TS in the UK prompted the release of guidance from the Royal College of Pediatrics and Child Health (RCPCH). 4 , 7 Following this, the WHO developed the definition for “multisystem inflammatory disorder in children and adolescents temporally related to COVID‐19” which was not very dissimilar from the RCPCH definition of PIMS‐TS. 10 All three cases in our report were very similar in their clinical presentation and laboratory findings, indicating a high likelihood of similar disease process. Each of the patients fulfilled both the RCPCH and WHO definitions. 7 , 10
Our findings in these three patients compare similarly to other cases across the UK, Europe and New York, United States of America (USA) which are all characterized by persistent fever and features of KD and/or TSS. 4 , 5 , 6 , 9 , 11 , 12 The age of those who were affected by this new disease entity can range from infancy to adolescence. 9 , 12 According to the European Centre for Disease Prevention and Control (ECDC) publication released on 14 May 2020, there were five deaths out of 250 PIMS‐TS cases. 9 This amounts to a mortality rate of approximately 2% but many of the reports did not specify death outcome. 9
PIMS‐TS is more prevalent among children from African origin as observed in the UK and France. 4 , 11 This observation can possibly be explained by disparity in socioeconomic status, poor and overcrowded living conditions and/or genetic susceptibility. On the cardiovascular involvement of PIMS‐TS, Africans may be of higher risk of COVID‐19‐related cardiac complications and death because of an intrinsic genetic susceptibility—a common SCN5A‐encoded Nav1.5 sodium channel variant p.Ser1103Tyr‐SCN5A—which predisposes the patients to ventricular arrhythmia and sudden cardiac death. 13 This is in addition to myocardial injury induced by cytokine storm, direct invasion of SARS‐CoV‐2 into cardiac myocytes, and hypoxic injury from accompanying respiratory compromise.
On the association of PIMS‐TS with recent SARS‐CoV‐2 infection, the first report from the UK (n = 8, mean age 8.9 ± 3.4 years) only had 2/8 (25%) cases of PIMS‐TS children with positive SARS‐CoV‐2 results but the authors did not mention the type of test or whether serological testing was employed. 4 Following that, the recent ECDC publication reported another larger study from the UK (n = 40, age range 11 months‐17 years), in which 12/37 (32%) children with PIMS‐TS had positive PCR tests and 17/20 (85%) were IgG positive. 9 In the Italian cohort (n = 10, mean age 7.5 ± 3.5 years), 2/10 (20%) patients with PIMS‐TS tested positive for SARS‐CoV‐2 by the PCR method, 8/10 (80%) had positive IgG results and 3/10 (30%) were IgM positive. 5 According to a recent publication from France (n = 21, median age 7.9 years, age range 3.7‐16.6 years), 8/21 (38%) of PIMS‐TS patients were PCR positive and 19/21 (90%) had positive SARS‐CoV‐2 IgG. 11 Among the 17 patients hospitalized for PIMS‐TS (n = 17, median age 8 years, age range 1.8‐16 years) in New York, USA, SARS‐CoV‐2 PCR was positive in 47% (8/17) and 9/17 (53%) were serologically positive. 12
These observations suggest that SARS‐CoV‐2 plays a causative role in the pathophysiology of PIMS‐TS and the convalescent SARS‐CoV‐2 IgG serological test may be a more reliable diagnostic test than PCR or IgM in children with PIMS‐TS. Also, the term “temporally associated with SARS‐CoV‐2” may be modified to make it more robustly associated with recent SARS‐CoV‐2 infection—as in this case series. Examining this phenomenon from the KD angle, studies done in the past have not convincingly established a causal link between human coronavirus and KD albeit possible association. 14 , 15
Although our patients had some signs and symptoms compatible with KD, for example, fever, rash, mucous membrane changes, conjunctivitis, cervical lymphadenopathy, thrombocytosis (in the later stage of disease), myocarditis, and coronary artery dilatation, there were differences from classical KD, such as being in an older age group, respiratory and gastrointestinal involvement, marked lymphopenia, thrombocytopenia and more severe clinical course. 4 , 5 , 11 , 12
KD is a type of vasculitis of unknown etiology which occurs more commonly in younger children. 16 KD is essentially a clinical diagnosis (characterized by fever, rash, non‐purulent conjunctivitis, cervical lymphadenopathy, oral and extremities changes) and there is no diagnostic test currently. 16 It can also present atypically in the form of KD shock syndrome (KDSS) and rarely macrophage activation syndrome (MAS), mimicking PIMS‐TS. 16 It is postulated that KD may be a manifestation of a genetic predisposition towards an abnormal immune response to some specific infectious agents. 16
MAS is closely related to hemophagocytic lymphohistiocytosis (HLH) and is classified as rheumatologic HLH because of its common association with systemic‐onset juvenile idiopathic arthritis, systemic lupus erythematosus, KD, and autoinflammatory disorders. 17 HLH can also be triggered by viral infections, for example, Epstein‐Barr virus and influenza. 17 The understanding of MAS pathophysiology is mostly derived from what is known about familial HLH (FHL). Perforin 1 (PRF1), unc‐13 homolog D (UNC13D), syntaxin11 (STX11), and syntaxin binding protein 2 (STXBP2) genes are responsible for cytotoxic granule release by CD8+ T cells and NK cells. 17 Biallelic mutations of these genes have been implicated in the development of FHL. Impaired cytotoxicity, continual intracellular infection, and persistent antigen stimulation result in T lymphocyte and macrophage activation, leading to massive release of pro‐inflammatory cytokines, for example, interleukin‐1β (IL‐1β), IL‐6, IL‐18, tumor necrosis factor (TNF), and interferon (IFN)‐γ. 17 It is possible that SARS‐CoV‐2 infection might be the trigger to this cascade of events among patients who are genetically susceptible.
MAS complicating KD is also commoner in older age groups and it can result in multiorgan dysfunction. 18 According to a systematic review, frequent features found among KD children with MAS were fever, splenomegaly anemia, elevated erythrocyte sedimentation rate (ESR), liver transaminases, ferritin and triglyceride, thrombocytopenia, hypofibrinogenemia, hemophagocytosis picture from bone marrow, liver, splenic and lymph node biopsy. IL‐1, IL‐8, IL‐10, and IFN‐γ are usually high but TNF‐α, IL‐1b, IL‐2R, IL‐2, IL‐4, and IL‐6 are not consistently raised. 18 Of the 32 children with cardiac complications in this systematic review, all had coronary artery abnormalities (CAA), only 3% (1/32) had ventricular dilatation, 6% (2/32) had valvular abnormalities, and 12% (4/32) had pericardial effusion. 18
The incidence rate of KDSS varies from 2% to 7% but MAS in association with KD is much rarer (about 1%). 18 , 19 When compared with patients with classical KD, children with KDSS were older and they tend to have a prolonged period of fever, gastrointestinal symptoms, lack of response to IVIG, higher incidence of CAAs, aseptic meningitis and longer hospital stay. 19 , 20 , 21 KDSS patients are significantly associated with leukocytosis, neutrophilia, higher C‐reactive CRP, ESR, procalcitonin, creatine kinase‐MB (CK‐MB), troponin I, creatinine, hepatic enzymes, triglyceride, brain natriuretic peptide (BNP), IL‐6, IL‐10, TNF‐α, IFN‐γ, and D‐dimer levels, anemia, hypoalbuminemia, hyponatremia and hypokalemia. 19 , 20 , 21 The cardiac abnormalities seen in patients with KDSS include CAAs (20‐55%), reduction in ejection fraction (15‐60%), valvular regurgitation (20%), and pericardial effusion (40%). It is hypothesized that shock in KDSS is related to myocardial dysfunction, systemic capillary leak syndrome and immune dysregulation mediated by overproduction of cytokines. 19 , 20 , 21
In the New York cohort of PIMS‐TS children who had cytokine profiling, 16/17 (94%) had elevated IL‐6 and 8/17 (47%) had increased IL‐2R, IL‐18, and CXCL‐9 levels. 12 Only 3/17 (18%) and 2/17 (12%) developed mildly increased IFN‐γ and IL‐8 levels, respectively. TNF‐α, IL‐1b, IL‐2, IL‐4, IL‐5, and IL‐13 levels were normal. 12 Cardiac abnormalities among PIMS‐TS patients include CAAs (20‐40%), left ventricular dysfunction (50‐65%), valvular regurgitation (15‐40%) and pericardial effusion (40‐50%). 4 , 5 , 11 , 12
The striking similarities between KDSS and PIMS‐TS may suggest that they are actually one entity instead of two different conditions. An alternative hypothesis on the mechanism of PIMS‐TS is the development of MAS in the course of COVID‐19 in these children, akin to the MAS manifestation seen in adult severe COVID‐19 and/or KD complicated by MAS. We recommend that future studies on PIMS‐TS children should include cytokine profiling, immunological tests, and investigations to compare and contrast PIMS‐TS from KDSS and MAS to better understand the pathogenesis of PIMS‐TS.
Children presenting with PIMS‐TS require prompt and aggressive respiratory, antibiotic, vasopressin, and fluid therapy. They should be discussed early with specialists of appropriate expertize and experience for escalation of care and for the use of IVIG with or without anti‐inflammatory agents (corticosteroid, tocilizumab) and heparin. 7 , 9 Further research into the optimum management of this condition is ongoing. 7
However, it must be emphasized that in the vast majority of cases, COVID‐19 is a relatively mild illness in children. Cases of PIMS‐TS are very rare and will only affect a very small minority of children.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
This study was conceptualized by SB, JWT, MZ, and VR. KN, TK, KG, and PWB were involved in data collection. TK and KG prepared the first manuscript draft. KN and JWT made major revisions to the draft. All authors reviewed the final draft and approved the submitted manuscript for publication.
ACKNOWLEDGMENTS
This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Ng KF, Kothari T, Bandi S, et al. COVID‐19 multisystem inflammatory syndrome in three teenagers with confirmed SARS‐CoV‐2 infection. J Med Virol. 2020;92:2880–2886. 10.1002/jmv.26206
REFERENCES
- 1. World Health Organization . WHO coronavirus disease (COVID‐19) dashboard. https://covid19.who.int/. Updated 2020. Accessed June 3, 2020.
- 2. Public Health England . Coronavirus (COVID‐19) in the UK. https://coronavirus.data.gov.uk/. Updated 2020. Accessed June 6, 2020.
- 3. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review [published online ahead of print April 22, 2020]. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 4. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395:1607‐1608. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395:1771‐1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. New York City Health Department . 2020 health alert #13: pediatric multi‐system inflammatory syndrome potentially associated with COVID‐19. https://www1.nyc.gov/assets/doh/downloads/pdf/han/alert/2020/covid-19-pediatric-multi-system-inflammatory-syndrome.pdf. Updated 2020. Accessed May 16, 2020.
- 7. Royal College of Paediatrics and Child Health (RCPCH) . Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID‐19. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19. Updated 2020. Accessed May 16, 2020.
- 8. Viner RM, Whittaker E. Kawasaki‐like disease: emerging complication during the COVID‐19 pandemic. Lancet. 2020;395:1741‐1743. 10.1016/S0140-6736(20)31129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Centre for Disease Prevention and Control . Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS‐CoV‐2 infection in children. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf. Updated 2020. Accessed May 16, 2020.
- 10. World Health Organization . Multisystem inflammatory syndrome in children and adolescents with COVID‐19. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Updated 2020. Accessed May 19, 2020.
- 11. Toubiana J, Poirault C, Corsia A, et al. Kawasaki‐like multisystem inflammatory syndrome in children during the COVID‐19 pandemic in Paris, France: Prospective observational study. BMJ. 369, 2020:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID‐19 in previously healthy children and adolescents in New York city [published online ahead of print June 8, 2020]. JAMA. 2020. 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Genetic susceptibility for COVID‐19‐associated sudden cardiac death in African Americans [published online ahead of print May 5, 2020]. Heart Rhythm. 2020. 10.1016/j.hrthm.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191(4):499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shirato K, Imada Y, Kawase M, Nakagaki K, Matsuyama S, Taguchi F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol. 2014;86(12):2146‐2153. 10.1002/jmv.23950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927‐e999. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 17. Henderson LA, Cron RQ. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr Drugs. 2020;22(1):29‐44. 10.1007/s40272-019-00367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia‐Pavon S, Yamazaki‐Nakashimada MA, Baez M, Borjas‐Aguilar KL, Murata C. Kawasaki disease complicated with macrophage activation syndrome: a systematic review. J Pediatr Hematol Oncol. 2017;39(6):445‐451. 10.1097/MPH.0000000000000872 [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Zheng Q, Zou L, et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL‐6, IL‐10 and IFN‐gamma as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17(1):1‐018. 10.1186/s12969-018-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma L, Zhang YY, Yu HG. Clinical manifestations of Kawasaki disease shock syndrome. Clin Pediatr (Phila). 2018;57(4):428‐435. 10.1177/0009922817729483 [DOI] [PubMed] [Google Scholar]
- 21. Taddio A, Rossi ED, Monasta L, et al. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. 2017;36(1):223‐228. 10.1007/s10067-016-3316-8 [DOI] [PubMed] [Google Scholar]


