Abstract
Omega-3 fatty acids are unsaturated fatty acids thought to play a role in health and disease. They are known as essential fatty acids, as they cannot be synthesized in mammals. Omega-3 fatty acids have a beneficial effect on the secondary prevention of coronary artery disease and stroke and are essential for the development and function of the nervous system and the retina in man. Omega-3 fatty acids are thought to have immunomodulatory properties as they act as precursors to lipid mediators of inflammation which may limit or modulate the inflammatory response. Omega-3 fatty acids seem to prevent or attenuate experimental arthritis. They may have a beneficial effect in the treatment of rheumatoid arthritis. Clinical studies have shown that omega-3 fatty acids may have a modulatory effect on disease activity, namely on the number of swollen and tender joints. It appears that omega-3 fatty acids may modulate disease activity in rheumatoid arthritis.
Keywords: Omega-3 fatty acids, experimental arthritis, rheumatoid arthritis
INTRODUCTION
Omega-3 fatty acids are known for their multiple effects on health and disease. Omega-3 fatty acids have been shown to protect against cardiovascular disease,1 playing a role in the secondary prevention of myocardial infarction,2 having an anti-arrhythmic action3 and reducing inflammation.4,5 Omega-3 fatty acids play a role in the treatment of inflammatory bowel disease, and, if taken, may have a beneficial effect.6 Omega-3 fatty acids are tested for their therapeutic potential in multiple sclerosis.7–9 Omega-3 fatty acids have been shown to have immunomodulatory properties.10,11 In this review, the effect of omega-3 fatty acids on rheumatoid arthritis (RA) will be discussed.
Fatty acids are saturated, monounsaturated and polyunsaturated.12 The main physical difference between them is that saturated fatty acids are solid at room temperature, while unsaturated fatty acids are liquid. Two types of polyunsaturated fatty acids exist: omega-6 polyunsaturated fatty acids, and omega-3 polyunsaturated fatty acids. Omega-6 fatty acids are available mainly from vegetable oils. Three types of omega-3 fatty acids exist: linolenic acid available from vegetable oils, and eicosapentaenoic acid and docosahexaenoic acid, which must be obtained from marine sources. Linoleic acid, a precursor of the omega-6 series of fatty acids, and α linolenic acid, a precursor of the omega-3 series of fatty acids, are considered essential fatty acids, as they cannot be synthesized in mammals. The names of the fatty acids come from the number of double unsaturated bonds between carbon atoms in the fatty acid chain. Omega-3 fatty acids have their first double bond in the third carbon molecule from the CH3 methyl or n or ω end of the molecule, whereas omega-6 fatty acids have their first double bond at the sixth carbon molecule from the CH3 methyl or n or ω end of the fatty acid molecule.13 Omega-6 linoleic acid can be desaturated in certain plants to form α linolenic acid. Linoleic acid is mainly converted into arachidonic acid, whereas α linolenic acid is elongated and desaturated to form eicosapentaenoic acid and docosahexaenoic acid. Western diet is characterized by the presence of significantly more omega-6 than omega-3 fatty acids the ratio of omega-6/omega-3 reaching as high as 20–30.14,15
MECHANISM OF ACTION
Omega-3 fatty acids have anti-inflammatory action. Eicosanoids are synthesized from omega-6 and omega-3 fatty acids. Arachidonic acid and eicosapentaenoic acid compete for the cyclo-oxygenase and lipoxygenase enzymes for conversion into eicosanoids. Those derived from arachidonic acid are pro-inflammatory and pro-aggregatory, whereas those derived from omega-3 fatty acids are anti-inflammatory and inhibit platelet aggregation. The beneficial effects of omega-3 fatty acids are mediated by themselves as well as their metabolites, namely resolvins, protectins and maresins. The most studied omega-3 metabolites are resolvins, which are classified into two classes. Class D resolvins are products of docosahexaenoic acid and class E resolvins are products of eicosapentaenoic acid.16–18 These metabolites of omega-3 fatty acids compete with those of omega-6 to promote the resolution of the inflammatory cycle.19,20 They are thought to play a significant role in the attenuation of inflammation and regulation of autoimmunity.19,20
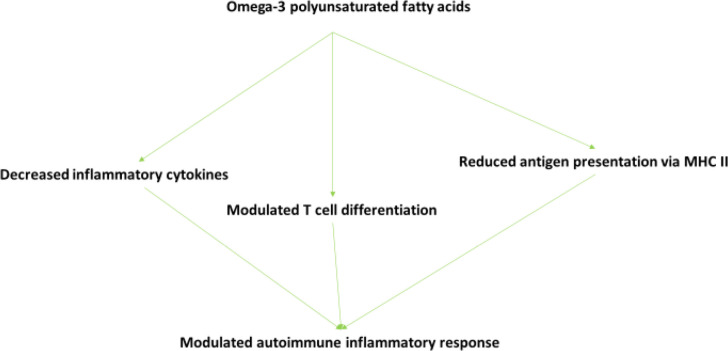
Omega-3 fatty acids may modulate proinflammatory cytokine secretion. In a study involving fish oil consumption by RA patients, the level of plasma interleukin-1β (IL-1β) decreased after fish oil consumption.21 In a clinical study in healthy volunteers, fish oil supplementation induced reduction of tumour necrosis factor-alpha (TNF-α), IL-1β, and interleukin-6 (IL-6) by endotoxin-stimulated monocytes cells.22 In studies with fish oil supplementation, it was observed that docosahexaenoic acid and eicosapentaenoic acid reduce the population CD4+ T cells which produce interferon-γ (IFN-γ) and interleukin-17 (IL-17).23 Studies in cultured cells have shown that eicosapentaenoic acid and docosahexaenoic acid inhibit the production of the well-known pro-inflammatory cytokines, namely, TNF-α, IL-1β and IL-6.24–27 In a transgenic strain of mice, the fat-1, in which endogenous production of omega-3 fatty acids is possible, inflammatory metabolites are markedly reduced.28,29 The suppression of inflammatory cytokines by omega-3 fatty acids may be a contributing factor to the amelioration of clinical signs and symptoms in RA. In other studies, eicosapentaenoic acid and docosahexaenoic acid inhibit the proliferation of human T cells in culture and the production of IL-2.30,31 Dietary omega-3 fatty acids have been shown to correct the imbalance in Th1 and Th2 ratios in RA and experimental autoimmune encephalitis.32,33 It appears, that proliferation and differentiation of T cells may be regulated by omega-3 fatty acids. In studies in vitro, the expression of major histocompatibility complex (MHC) II and antigen presentation via MHC II may be reduced following exposure to omega-3 fatty acids.34,35 These in vitro results have been confirmed in mice and in humans.
The above-mentioned data show that omega 3-fatty acids may decrease inflammatory cytokines, modulate T-cell differentiation and reduce antigen presentation via MHC II thus modulating the inflammatory autoimmune response ( Figure 1).
Figure 1.
Mode of action of omega-3 polyunsaturated fatty acids on the autoimmune inflammatory response (MHC -major histocompatibility complex).
EXPERIMENTAL FINDINGS
Studies performed in animal models have shown a protective effect of omega-3 fatty acids against experimentally induced arthritis. Fish-oil feeding in mice delayed the onset and reduced the incidence and severity of type II collagen-induced arthritis compared with the vegetable oil-fed group.36 In the DBA/1 mouse strain, which is susceptible to the development of collagen-induced arthritis, daily intake of marine omega-3 polyunsaturated fatty acids in the form of phospholipids delayed the onset of arthritis, decreased the severity, reduced paw swelling, and knee joint pathology in collagen-induced arthritis.37 In a rat model, eicosapentaenoic acid and docosahexaenoic acid have been shown to suppress Streptococcal-induced arthritis.38 In this model, eicosapentaenoic acid appeared to be more effective than docosahexaenoic acid. Endogenous production of omega-3 fatty acids in the fat-1 transgenic mice drastically attenuated arthritis as well as local and systemic levels of inflammatory cytokines following the establishment of RA, whereas the wild type control mice developed overt arthritis.39 Docosahexaenoic acid has been shown to ameliorate experimentally induced autoimmune encephalitis in mice.40 It appears that dietary-induced changes of tissue levels of polyunsaturated fatty acids modify inflammatory reactions through changes in the synthesis of lipid mediators of inflammation ( Figure 2).38
Figure 2.
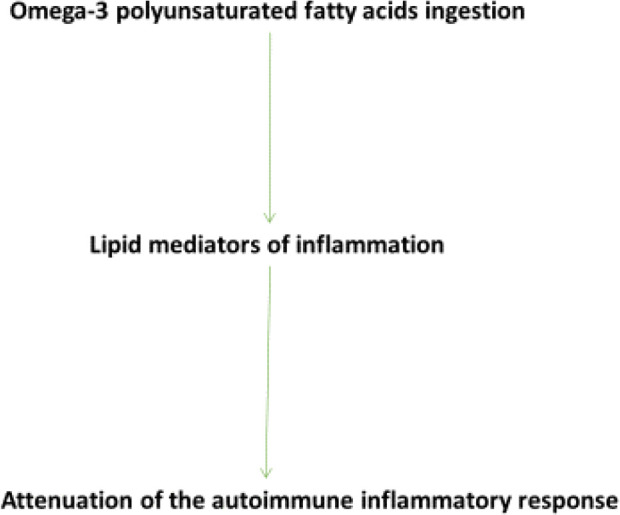
The effect of dietary omega-3 polyunsaturated fatty acids on the autoimmune inflammatory response.
CLINICAL FINDINGS
In an early study published in the Lancet in 1985, Kremer et al.41 investigated the effect of diet manipulation in RA patients. In a 12-week, prospective, double-blind controlled study, 17 patients received an experimental diet high in polyunsaturated and low in saturated fat, with a daily supplement of 1.8 g eicosapentaenoic acid, while 20 patients received a control diet with a lower polyunsaturated:saturated fat ratio and a placebo supplement. The experimental group at 12 weeks had lower levels of morning stiffness and lower number of tender joints. During follow-up and 1–2 months after stopping the diet, the experimental group had deteriorated significantly. In a subsequent study, Kremer et al.42 investigated the effect of omega-3 fatty acids on RA patients. In a 24-week, prospective, randomized, double-blind study involving 49 patients with RA, they studied the effect of high dose fish oil (dietary supplements with 54 mg/kg eicosapentaenoic acid and 36 mg/kg docosahexaenoic acid), low dose fish oil (27 mg/kg eicosapentaenoic acid and 18 mg/kg docosahexaenoic acid) and olive oil on the disease. They observed significant decrease in the number of tender and swollen joints in both groups with omega-3 fish oil supplements, the effect observed earlier in the high dose group. Cytokine production by immune cells also decreased. Namely, neutrophil leukotriene B4 production and macrophage interleukin-1 production decreased significantly after fish oil supplementation. Veselinovic et al.43 investigated the effect of omega-3 fatty acid supplementation in 60 patients with RA in a 12-week prospective, randomized trial. Patients received omega-3 fatty acids, omega-3 fatty acids plus evening primrose oil or no supplement. They observed a significant decrease in DAS28, number of tender joints and VAS score in both experimental groups. In a study performed in Sweden involving 737 patients with early RA, omega-3 fatty acid intake was associated with a good response to treatment according to the EULAR criteria.44 In a prospective, controlled, double-blind trial performed in Iran involving 61 RA patients, omega-3 fatty acids were administered to the patients along with their standard treatment.11 The patients were assessed and significant improvement was noted in the patient’s global evaluation, and in the physician’s assessment of disease in those taking omega-3. The proportions of patients who improved and of those who were able to reduce their concomitant analgesic medication were significantly greater with omega-3 consumption, while no weight change was observed. In a randomized, controlled, double-blind study performed in Austria involving 23 patients with active RA, patients received an infusion of fish oil emulsion for 14 days followed by oral treatment with fish oil capsules.45 Swollen joint count was significantly lower in the omega-3 fatty acid group after 1 and 2 weeks of infusion. Tender joint count tended also to be lower in the omega-3 fatty acid group after 1 and 2 weeks of infusion. Both swollen and tender joint counts were significantly lower in the omega-3 fatty acid group on oral treatment compared to the placebo group. In a food frequency study performed in Norway involving 78 RA patients, Beyer et al.46 found that seafood intake according to nutritional recommendations was related to better disease outcome. In a study performed in the Netherlands, van der Tempel et al.47 investigated the effect of fish oil supplementation on RA and they observed a decrease in morning stiffness and joint swelling index. In a study involving 32 patients with RA, fish oil was administered and plasma interleukin-1β levels decreased.21 Lee et al.48 performed a meta-analysis on the effect of omega-3 fatty acids on clinical outcomes in RA. Their meta-analysis included 10 randomized controlled trials involving 187 RA patients and 183 placebo-treated RA control subjects. They showed that omega-3 fatty acid consumption reduced NSAIDs consumption without between-study heterogeneity. Tender joint count, swollen joint count, morning stiffness and physical function tended to improve more in patients receiving omega-3 fatty acids, but the effect did not reach statistical significance. Thus, it appears that omega-3 fatty acids may have an anti-inflammatory action and may decrease disease activity in RA.49 In the era of precision medicine,50 precision nutrition51 is a new concept which may modulate the autoimmune response52 and may aid in the management of RA.
CONCLUSION
Omega-3 fatty acids are polyunsaturated fatty acids which have an impact in health and disease. They act as precursors to lipid mediators of inflammation and may attenuate and modulate the autoimmune inflammatory response. They have been shown to ameliorate or prevent experimental arthritis and may decrease disease activity in rheumatoid arthritis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 1985;312(19):1205–9. [DOI] [PubMed] [Google Scholar]
- 2.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem 2004;263(1–2):217–25. [DOI] [PubMed] [Google Scholar]
- 3.Galano JM, Roy J, Durand T, Lee J, Le Guennec J-Y, Oger C, et al. Biological activities of non-enzymatic oxygenated metabolites of polyunsaturated fatty acids (NEO-PUFAs) derived from EPA and DHA: New anti-arrhythmic compounds? Mol Aspects Med 2018;64:161–8. [DOI] [PubMed] [Google Scholar]
- 4.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 2018;132:41–8. [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 2017;45(5):1105–15. [DOI] [PubMed] [Google Scholar]
- 6.Ungaro F, Rubbino F, Danese S, D’Alessio S. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases. Front Immunol 2017;8:1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbige LS, Sharief MK. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. Br J Nutr 2007;98 Suppl 1:S46–53. [DOI] [PubMed] [Google Scholar]
- 9.Riccio P. The molecular basis of nutritional intervention in multiple sclerosis: a narrative review. Complement Ther Med 2011;19(4):228–37. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC. Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes. Mar Drugs 2019;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajaei E, Mowla K, Ghorbani A, Bahadoram S, Bahadoram M, Dargahi-Malamir M. The Effect of Omega-3 Fatty Acids in Patients With Active Rheumatoid Arthritis Receiving DMARDs Therapy: Double-Blind Randomized Controlled Trial. Glob J Health Sci 2015;8(7):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease--fishing for a natural treatment. BMJ 2004;328(7430):30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010;68(5):280–9. [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet 2003;92:1–22. [DOI] [PubMed] [Google Scholar]
- 15.Kang JX. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev Nutr Diet. 2003;92:23–36. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol 2006;176(3):1848–59. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 2000;192(8):1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278(17):14677–87. [DOI] [PubMed] [Google Scholar]
- 19.Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013;62(6):1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson JE, Kim JS, Das A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat 2019;143:106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espersen GT, Grunnet N, Lervang HH, Nielsen GL, Thomsen BS, Faarvang KL, et al. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin Rheumatol 1992;11(3):393–5. [DOI] [PubMed] [Google Scholar]
- 22.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996;63(1):116–22. [DOI] [PubMed] [Google Scholar]
- 23.Ye P, Li J, Wang S, Xie A, Sun W, Xia J. Eicosapentaenoic acid disrupts the balance between Tregs and IL-17+ T cells through PPARgamma nuclear receptor activation and protects cardiac allografts. J Surg Res 2012;173(1):161–70. [DOI] [PubMed] [Google Scholar]
- 24.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol 2003;284(1):L84–89. [DOI] [PubMed] [Google Scholar]
- 25.Babcock TA, Novak T, Ong E, Jho DH, Helton WS, Espat NJ. Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-alpha production by omega-3 fatty acid is associated with differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J Surg Res 2002;107(1):135–9. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr 2004;23(1):71–8. [DOI] [PubMed] [Google Scholar]
- 27.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, et al. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant cosupplementation. Br J Nutr 2003;90(2):405–12. [DOI] [PubMed] [Google Scholar]
- 28.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004;427(6974):504. [DOI] [PubMed] [Google Scholar]
- 29.Wei D, Li J, Shen M, Jia W, Chen N, Chen T, et al. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 2010;59(2):471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pompos LJ, Fritsche KL. Antigen-driven murine CD4+ T lymphocyte proliferation and interleukin-2 production are diminished by dietary (n-3) polyunsaturated fatty acids. J Nutr 2002;132(11):3293–3300. [DOI] [PubMed] [Google Scholar]
- 31.Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, et al. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Invest 2017;127(5):1757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizota T, Fujita-Kambara C, Matsuya N, Hamasaki S, Fukudome T, Goto H, et al. Effect of dietary fatty acid composition on Th1/Th2 polarization in lymphocytes. JPEN J Parenter Enteral Nutr 2009;33(4):390–6. [DOI] [PubMed] [Google Scholar]
- 33.Sierra S, Lara-Villoslada F, Comalada M, Olivares M, Xaus J. Dietary fish oil n-3 fatty acids increase regulatory cytokine production and exert anti-inflammatory effects in two murine models of inflammation. Lipids 2006;41(12):1115–25. [DOI] [PubMed] [Google Scholar]
- 34.Khair-el-Din TA, Sicher SC, Vazquez MA, Wright WJ, Lu CY. Docosahexaenoic acid, a major constituent of fetal serum and fish oil diets, inhibits IFN gamma-induced Ia-expression by murine macrophages in vitro. J Immunol 1995;154(3):1296–306. [PubMed] [Google Scholar]
- 35.Hughes DA, Pinder AC. N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin Exp Immunol 1997;110(3):516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie CA, Gonnerman WA, Ullman MD, Hayes KC, Franzblau C, Cathcart ES. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med 1985;162(4):1336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ierna M, Kerr A, Scales H, Berge K, Griinari M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet Disord 2010;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volker DH, FitzGerald PE, Garg ML. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN rats. J Nutr 2000;130(3):559–65. [DOI] [PubMed] [Google Scholar]
- 39.Woo SJ, Lim K, Park SY, Jung MY, Lim HS, Jeon MG, et al. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids attenuates K/BxN serum-transfer arthritis in fat-1 mice. J Nutr Biochem 2015;26(7):713–20. [DOI] [PubMed] [Google Scholar]
- 40.Adkins Y, Soulika AM, Mackey B, Kelley DS. Docosahexaenoic acid (22:6n-3) Ameliorated the Onset and Severity of Experimental Autoimmune Encephalomyelitis in Mice. Lipids 2019;54(1):13–23. [DOI] [PubMed] [Google Scholar]
- 41.Kremer JM, Bigauoette J, Michalek AV, Timchalk MA, Lininger L, Rynes RI, et al. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet 1985;1(8422):184–7. [DOI] [PubMed] [Google Scholar]
- 42.Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum 1990;33(6):810–20. [DOI] [PubMed] [Google Scholar]
- 43.Veselinovic M, Vasiljevic D, Vucic V, Arsic A, Petrovic S, Tomic-Lucic A, et al. Clinical Benefits of n-3 PUFA and -Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients 2017;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lourdudoss C, Wolk A, Nise L, Alfredsson L, Vollenhoven RV. Are dietary vitamin D, omega-3 fatty acids and folate associated with treatment results in patients with early rheumatoid arthritis? Data from a Swedish population-based prospective study. BMJ Open 2017;7(6):e016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahadori B, Uitz E, Thonhofer R, Trummer M, Pestemer-Lach I, McCarty M, et al. omega-3 Fatty acids infusions as adjuvant therapy in rheumatoid arthritis. JPEN J Parenter Enteral Nutr 2010;34(2):151–5. [DOI] [PubMed] [Google Scholar]
- 46.Beyer K, Lie SA, Kjellevold M, Dahl L, Brun JG, Bolstad AI. Marine ω-3, vitamin D levels, disease outcome and periodontal status in rheumatoid arthritis outpatients. Nutrition 2018;55–56:116–24. [DOI] [PubMed] [Google Scholar]
- 47.van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis 1990;49(2):76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res 2012;43(5):356–62. [DOI] [PubMed] [Google Scholar]
- 49.Gioxari A, Kaliora AC, Marantidou F, Panagiotakos DP. Intake of omega-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018;45:114–124. e114. [DOI] [PubMed] [Google Scholar]
- 50.Beckmann JS, Lew D. Reconciling evidence-based medicine and precision medicine in the era of big data: challenges and opportunities. Genome Med 2016;8(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chilton FH, Dutta R, Reynolds LM, Sergeant S, Mathias RA, Seeds MC. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients 2017;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ. Therapeutic Potential of omega-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front Immunol 2019;10:2241. [DOI] [PMC free article] [PubMed] [Google Scholar]