The optimal treatment for patients with newly diagnosed acute myeloid leukaemia (AML) who are infected with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2)/coronavirus disease 2019 (COVID‐19) is unknown. 1 We report the case of a previously fit 27‐year‐old male who presented with a 3‐day history of fever (>39°C), swollen, erythematous elbows and no respiratory symptoms. His white blood cell count (WBC) was 187 × 109/l and bone marrow (Fig 1A,C) examination revealed normal karyotype AML with a fms related receptor tyrosine kinase 3 (FLT3) internal tandem duplication (ITD), wild‐type nucleophosmin 1 (NPM1) and no additional mutations on a next‐generation sequencing panel.
Fig 1.
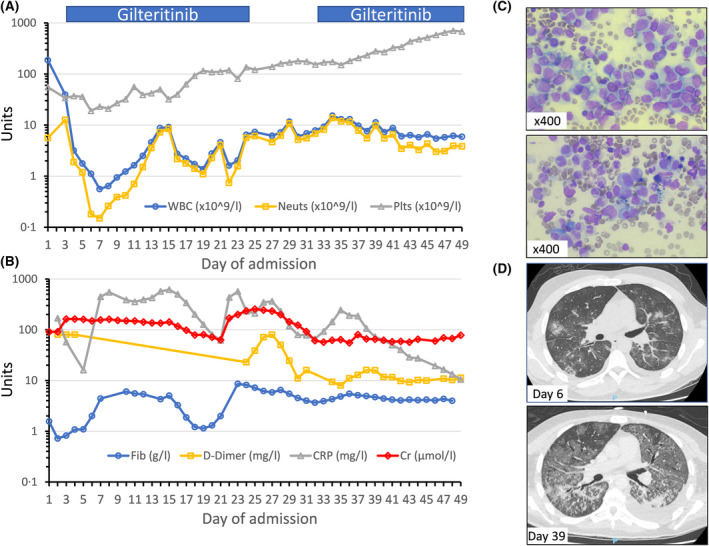
Full blood count parameters are shown in Panel A; white blood cell count (WBC, reference range 3–10 × 109/l), neutrophils (2–7·5 × 109/l) and platelets (150–400 × 109/l). Gilteritinib administration (120 mg once daily), starting from day 3 onwards, is indicated by blue bars. The patient presented with high D‐dimers >80 mg/l (range <0.5 mg/l; Panel B) and hypofibrinogenaemia (range 1·5–4 g/l), followed by a hyperfibrinogenaemic stage during which C‐reactive protein (CRP; range <5 mg/l, Panel B) peaked. Mild tumour lysis syndrome developed with a near doubling of baseline creatinine (range 66–112 µmol/l; Panel B). Morphological analysis of the bone marrow smear at diagnosis (Panel C, top pane) showed heavy infiltration by myelomonocytic blasts, which were positive for CD34, CD117, human leucocyte antigen DR isotype (HLA‐DR), CD33, CD15, CD38, cytoplasmic myeloperoxidase and weakly positive for CD7 by flow cytometry (not shown). The post‐induction bone marrow smear showed morphological (Panel C, bottom pane) and flow cytometric complete remission. The initial high‐resolution CT (HRCT) scan on day 6 (Panel D, top pane) displays patchy infiltrates with extensive patchy areas of ground glass opacification and ‘crazy paving’ pattern, typical of severe COVID‐19. Repeat HRCT at day 39 (bottom pane) showed widespread ground glass opacification, as well as areas of consolidation and a progressive left‐sided pleural effusion.
Hyperleucocytosis was immediately treated with hydroxycarbamide, dexamethasone, rasburicase (for tumour lysis syndrome prophylaxis), and three doses of 100 mg/m2 cytarabine over the first 48 h. He received antibiotics to treat febrile neutropenia and cellulitis and although he denied any respiratory symptoms, a combined nasal and pharyngeal swab for SARS‐CoV‐2 RNA was positive. Doppler ultrasound revealed a lower‐limb deep vein thrombosis, bilateral upper‐arm superficial thrombophlebitis and coagulation markers were indicative of disseminated intravascular coagulation (Fig 1B). Given his active SARS‐CoV‐2 infection and the presence of a FLT3‐ITD mutation, he was treated with single‐agent gilteritinib, an oral FLT3 inhibitor, 2 from day 3. Gilteritinib shows superior efficacy to salvage chemotherapy in relapsed/refractory FLT3‐mutated AML, 3 with low rates of infection and early mortality, although it is not currently licensed for use in de novo AML.
On day 6, he became hypoxic, with hyperpyrexia, rising C‐reactive protein (CRP; Fig 1B), and a high‐resolution computed tomography (HRCT) scan of the chest (Fig 1D) showed changes typical for COVID‐19. He was transferred to the intensive care unit on day 7 for continuous positive airway pressure (CPAP) support, but deteriorated further on day 13, requiring emergency intubation due to adult respiratory distress syndrome. Dexamethasone was briefly restarted on day 14 to preventatively treat early differentiation syndrome. 2 He was extubated to CPAP on day 20; however, he experienced a febrile episode associated with seizures on day 22 due to Escherichia coli bacteraemia, which precipitated re‐intubation, vasopressor support and further antibiotics. Gilteritinib was temporarily discontinued for 7 days from day 25 (Fig 1A) due to biochemical features of septic shock‐related cardiomyopathy.
During the admission, the patient experienced only 5 days of severe neutropenia (<0·5 × 109/l) and 17 days of thrombocytopenia (<50 × 109/l). Post‐induction bone marrow examination showed morphological (Fig 1C) complete remission and a significant reduction in FLT3‐ITD allele ratio from 0·66 at diagnosis to 0·07. He received a tracheostomy without incident on day 33. A repeat HRCT on day 39 (Fig 1D) showed extensive changes associated with severe COVID‐19. He was decannulated on day 45 and transferred back to the ward for intensive rehabilitation. SARS‐CoV‐2 RNA remained detectable on weekly nasopharyngeal swabs until day 60 when it became undetectable. At the time of writing, he continues on gilteritinib, with a plan to proceed to allogeneic stem cell transplantation when he is physically fit.
Though the effects of SARS‐CoV‐2 infection on patients with AML are largely unknown, 1 early evidence suggests that patients with active haematological malignancies and COVID‐19 have more severe disease and a higher case fatality rate. 4 , 5 Provisional guidance 1 , 6 recommends delaying AML induction chemotherapy in patients with concurrent COVID‐19, an option not possible in this case. Induction mortality rates with intensive chemotherapy in those with hyperleucocytosis can approach 30% and such chemotherapy is associated with prolonged pancytopenia (often >3 weeks) and high rates of severe infections. 7 We conclude that single‐agent gilteritinib can be safely administered and induce remission in patients presenting with de novo FLT3‐ITD‐positive AML. Although further studies are required in this setting, gilteritinib can be considered as a treatment option for patients with FLT3‐mutated AML and severe COVID‐19, where a prolonged period of chemotherapy‐induced pancytopenia could adversely affect outcomes.
Conflicts of interest
Andrew J. Wilson: personal fees from Novartis; Marc R. Mansour: advisory boards for Janssen; Elspeth Payne: advisory boards for Novartis, Celegene and Takeda; Asim Khwaja: personal fees from Astellas, outside the submitted work.
The other authors have no conflicts of interest to declare.
Acknowledgements
Andrew J. Wilson, Ethan Troy‐Barnes, Maryam Subhan, Fiona Clark, Rajeev Gupta, Adele K. Fielding, Panagiotis Kottaridis, Marc R Mansour, Jenny O'Nions, Elspeth Payne, Kirsty Thomson and Asim Khwaja provided clinical care for the patient and were involved in critically revising the manuscript. Naina Chavda and Robert Baker provided expert assistance in diagnostics and critically revising the manuscript. All authors have approved the final manuscript.
References
- 1. Paul S, Rausch C, Jain N, Kadia T, Ravandi F, DiNardoet CD, et al. Treating leukemia in the time of COVID‐19. Acta Haematol. 2020;1–13. 10.1159/000508199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perl, AE , Martinelli, G , Cortes, JE , Neubauer, A , Berman, E & Paolini, S , et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3‐mutated AML. N Engl J Med. 2019;381:1728–40. [DOI] [PubMed] [Google Scholar]
- 4. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020;34:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martín‐Moro, F. , Marquet, J. , Piris, M. , Michael, B.M. , Sáez, A.J. & Corona, M. et al. Survival study of hospitalized patients with concurrent Covid‐19 and haematological malignancies. Br J Haematol. 2020;190 1:e16–20. 10.1111/bjh.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCRI AML Working Party . Recommendations for the management of patients with AML during the COVID19 outbreak: a statement from the NCRI AML Working Party [online]. 2020. Available from: http://www.cureleukaemia.co.uk/page/news/523/aml-working-party-covid-19-recommendations. Accessed 09/05/2020.
- 7. Nørgaard M, Larsson H, Pedersen G, Schønheyder HC, Sørensen HT. Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect. 2006;12:217–23. [DOI] [PubMed] [Google Scholar]


