Dear Editor,
I read with interest the recent published article from Professor Robert L. Medcalf, entitled “Fibrinolysis and COVID‐19: a plasmin paradox.”1 As an indirect marker of thrombin and plasmin activation, D‐dimer has been suggested to guide anticoagulant treatment in COVID‐19 patients.2., 3. However, D‐dimer may not be able to reflect accurate fibrinolysis status of COVID‐19 patients, and therefore can't guide the possible antifibrinolysis or thrombolytic therapy in different stages of COVID‐19, as Professor Medcalf discussed. Hence, we speculated that measuring direct markers of thrombin, plasmin, and so on may provide more therapeutic targets in COVID‐19 patients with coagulopathy.
To describe the intuitive coagulation and fibrinolysis features of COVID‐19 patients, we randomly enrolled 20 patients with critical COVID‐19 entering the intensive care unit (ICU) of Tongji Hospital in Wuhan, China, from February 1 to February 20, 2020; all of these patients stayed in ICU for 15 to 20 days and received a prophylactic dose of low molecular weight heparin (LMWH) for at least 7 days. Their residual plasma samples for routine coagulation tests during ICU stay were reserved at −70 degrees. Recently, we detected the levels of thrombin‐antithrombin complex (TAT), plasmin‐antiplasmin complex (PAP), and tissue plasminogen activator‐plasminogen activator inhibitor 1 complex (tPAI‐C) of these samples using a HISCL 5000 analyzer and original chemiluminescence reagents (SYSMEX). Levels of these three markers reflect activities of thrombin, plasmin, and plasminogen activator inhibitor‐1 (PAI‐1), respectively.
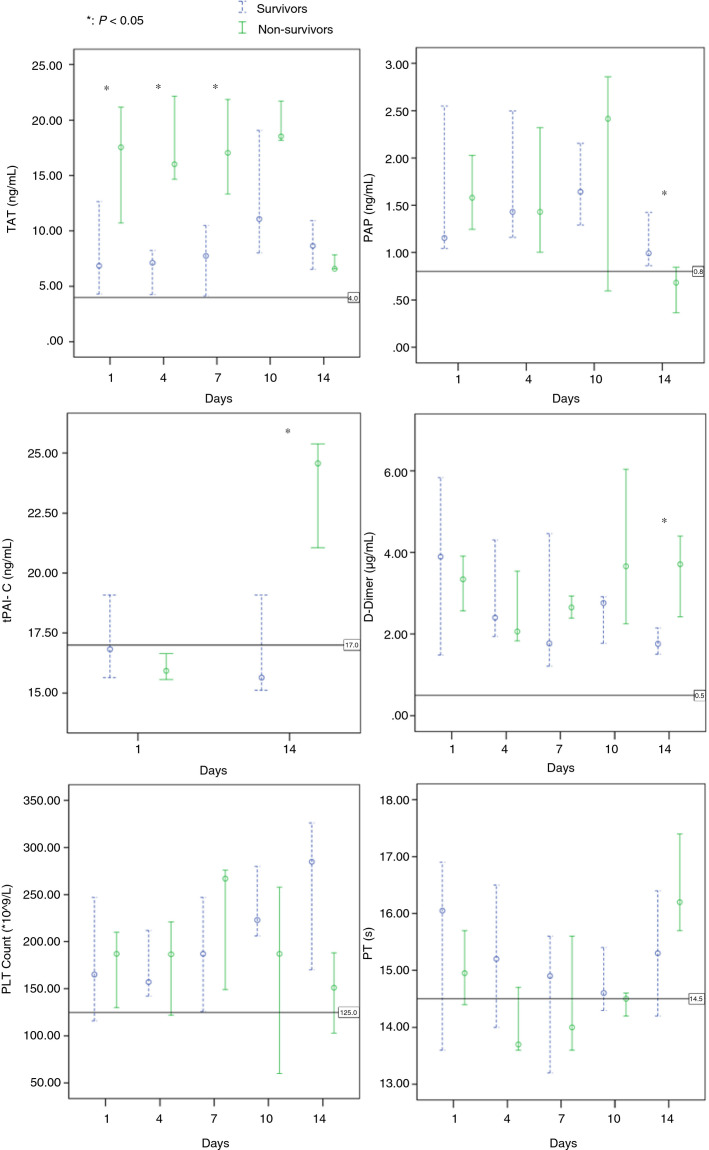
Eventually, 8 patients (40%) died and 12 patients were discharged. The results of D‐dimer, prothrombin time (PT), platelet count, TAT, and PAP on days 1, 4, 7, 10, and 14 between survivors and non‐survivors were compared (Figure 1 ). In addition, the results of tPAI‐C on days 1 and 14 between survivors and non‐survivors were also compared.
Figure 1.
Dynamic profile of coagulation parameters in critical COVID‐19 patients. Timeline charts illustrate the changes of coagulation parameters in 20 critical patients with COVID‐19 (8 non‐survivors and 12 survivors) during intensive care unit stay. The error bars show medians and 25% and 75% percentiles. The horizontal lines show the upper normal limits of thrombin‐antithrombin complex (TAT; 4.0 ng/mL), plasmin‐antiplasmin complex (PAP; 0.8 µg/mL), tissue plasminogen activator‐plasminogen activator inhibitor 1 complex (TPAI‐C; 17.0 ng/mL), D‐dimer (0.5 µg/mL), and prothrombin time (PT; 14.5 s), and the lower normal limits of platelet count (125 × 109/L), respectively. *, P < .05 for survivors versus non‐survivors with Mann‐Whitney U test
Perhaps due to the fact that LMWH was routinely used in all of the enrolled patients, no significant difference on results of D‐dimer, PT, and platelet count during the early and middle stage were found between survivors and non‐survivors. Three (37.5%) of the non‐survivors met the International Society on Thrombosis and Haemostasis (ISTH) diagnostic criteria for disseminated intravascular coagulation (DIC) during ICU stay; this incidence rate was also lower than that in our previous study (71.4%, P < .05).4
Interestingly, the other specific coagulation markers we detected might provide more therapeutic targets: the higher TAT levels in non‐survivors than in survivors during the early and middle stage reflected more excess generation of thrombin,5 and might indicate higher dose of anticoagulant; the higher tPAI‐C levels in non‐survivors than in survivors during the late stage reflected fibrinolysis shutdown due to endothelial dysfunction,6 and might indicate further thrombolytic therapy with tissue plasminogen activator.
Although D‐dimer levels in non‐survivors increased significantly at the late stage, the PAP levels in them were decreased and significantly lower than survivors; this perhaps implies a hypofibrinolysis status due to increased PAI‐1 (reflected by tPAI‐C level) as well as excess consumption of plasminogen.7 Hence, PAP levels could reflect more accurate fibrinolytic status than D‐dimer at the late stage, and avoid unnecessary (even harmful) anti‐fibrinolytic therapy in critical COVID‐19 patients. In addition, as Professor Medcalf mentioned in his article, if an antifibrinolytic agent such as tranexamic acid was to be given early to COVID‐19 patients for inhibiting infectivity of coronavirus, at what point would this need to be stopped? We consider that PAP level may be used to guide the antifibrinolysis therapy with appropriate thresholds.
Our study was retrospective and with small sample size, the results should be confirmed in an adequately powered intervention study. As the mortality seems still high in critical COVID‐19 patients receiving prophylactic anticoagulant, whether treatment strategies based on these specific coagulation markers could further improve outcome of critical COVID‐19 patients was the issue worthy of investigation.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Funding Information
National Mega Project on Major Infectious Disease Prevention of China (No. 2017ZX10103005‐007).
Acknowledgments
National Mega Project on Major infectious Disease Prevention of China 2017ZX10103005‐007
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 25 June 2020
REFERENCES
- 1.Medcalf R.L., Keragala C.B., Myles P.S. Fibrinolysis and COVID‐ 19: a plasmin paradox. J Thromb Haemost. 2020 doi: 10.1111/jth.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., et al. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gando S., Nanzaki S., Sasaki S., Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79(6):1111–1115. [PubMed] [Google Scholar]
- 6.Schmitt F.C.F., Manolov V., Morgenstern J., et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semeraro N., Ammollo C.T., Semeraro F., Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]