Abstract
On 11 March 2020, the World Health Organization (WHO) has declared the novel coronavirus disease (COVID‐19) a global pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2 virus). A consistent number of case reports and clinical series have been already published describing a complex spectrum of skin manifestations associated with the SARS‐CoV‐2 infection. We carried out a review of the English‐language literature up to 20 May 2020, reporting original cases or case series of the cutaneous manifestations of SARS‐CoV‐2 virus infection. The following databases were consulted: PubMed, Embase, Google Scholar and ResearchGate. The search of papers was conducted by using the key term ‘COVID‐19’ or ‘SARS‐CoV‐2’ or ‘coronavirus’ combined with each of the following: ‘skin’, ‘cutaneous’, ‘dermatologic’ or ‘dermatology’, ‘manifestation’, ‘lesions’, or ‘rash’. The patterns of dermatological manifestations associated with SARS‐CoV‐2 infection could be classified into four categories: exanthema (varicella‐like, papulo‐vesicular and morbilliform rash), vascular (chilblain‐like, purpuric/petechial and livedoid lesions), urticarial and acro‐papular eruption. Lastly, other skin manifestations to be considered are the cutaneous adverse reactions to the drugs prescribed for the treatment of COVID‐19. Whether SARS‐CoV‐2 infection can directly cause a worsening of chronic inflammatory diseases such as psoriasis or atopic dermatitis remains to be determined. Dermatology's outlook in the COVID‐19 pandemic is multidimensional.
Introduction
On 11 March 2020, the World Health Organization (WHO) has declared the novel coronavirus disease (COVID‐19) a global pandemic. 1 The disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2 virus) was firstly identified in Wuhan, China, in December 2019 and with a rapid evolving scenario has become a serious threat to global public health. 2 As of 20 May 2020, more than 4 900 000 cases of COVID‐19 have been reported, with more than 320 000 deaths. Transmission of SARS‐CoV‐2 mainly occurs in households and other close settings, including nosocomial ones. The virus enters the body through mucosal surfaces via droplets, aerosols or hand contact. Disease presentation can range from no symptoms to acute respiratory distress syndrome (ARDS), multiple organ failure and death. The most common symptoms include fever, dry cough, fatigue, sputum production, shortness of breath, loss of smell and taste, and conjunctivitis. Severe disease is characterized by dyspnoea, blood oxygen desaturation, respiratory failure and venous thromboembolism. 2 , 3 As a new emerging virus infection, the dermatological manifestations associated with SARS‐CoV‐2 infection are an important issue to consider. The conspicuous numbers of case reports and clinical series that have been recently published have described a complex spectrum of skin manifestations associated with the infection. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 The aim of this review is to summarize the major patterns of dermatological manifestations associated with SARS‐CoV‐2 virus infection.
Materials and methods
We carried out a review of the English‐language literature up to 20 May 2020, related to the cutaneous manifestation of SARS‐CoV‐2 virus infection. The following databases were consulted: PubMed, Embase, Google Scholar and ResearchGate. The research of articles was conducted by using the key term ‘COVID‐19’ or ‘SARS‐CoV‐2’ or ‘coronavirus’ combined with each of the following: ‘skin’, ‘cutaneous’, ‘dermatologic’ or ‘dermatology’, ‘manifestation’, ‘lesions’, or ‘rash’. For each article, the clinical pattern, type of elementary lesions, localization, early or late onset, age, associated symptoms and number of cases reported were collected.
Results
The patterns of dermatological manifestations associated with SARS‐CoV‐2 infection are reported in Table 1. The cutaneous manifestations could be classified into the following patterns: exanthema (varicella‐like, papulo‐vesicular and morbilliform rash), vascular (chilblain‐like, purpuric/petechial and livedoid lesions), urticarial and acro‐papular eruption. Each pattern is described below.
Table 1.
Pattern of dermatological lesions associated with COVID‐19
| Pattern | Subtype | Localization | Onset | Age | Symptoms |
|---|---|---|---|---|---|
| Exanthema | Varicella‐like papulo‐vesicular | Trunk ± limbs | Early | Mostly adult | Pruritus |
| Morbilliform rash | Trunk ± limbs | Early | Adult | Pruritus | |
| Vascular | Chilblain‐like lesions | Digits | Late | Mostly child | Pruritus |
| Purpuric/petechial lesions | Trunk, buttock, limbs | Late | Adult | Burning | |
| Livedoid lesions | Limbs | Late | Adult | None | |
| Urticaria | Weals | Face, upper body | Early | Adult | Pruritus, fever |
| Acro‐papular | Erythematous papules | Limbs | Late | Adult | Pruritus |
Exanthema pattern
Varicella‐like exanthema
The varicella‐like exanthema was firstly described by Marzano et al. 13 and Galván Casas et al. 14 as a specific COVID‐19‐associated skin manifestation. It is clinically characterized by widespread monomorphic papulo‐vesicular lesions. In the recent prospective nationwide study conducted in Spain, 14 this exanthema was described in 9% of the 375 patients studied. The lesions appear at a median of 3 days after symptoms and last for a median time of 8 days. It is associated with moderate disease severity generally in middle‐aged patients. Trunk is constantly involved, and itching is reported in some, but not all patients.
Histology shows vacuolar degeneration with disorganized keratinocytes, enlarged and multinucleate keratinocytes with dyskeratotic (apoptotic) cells. A dense inflammatory infiltrate may be present.
Maculo‐papular exanthema
A maculo‐papular rash, defined also as morbilliform, presenting clinical features that resemble typical viral exanthemas has been also described in COVID‐19 patients. 15 , 16 , 17 , 18 It appears concomitantly with the other symptoms of infection and lasts for a short period (3–10 days), and itching is reported in most of the patients. It is associated with severe disease in older patients. Galván Casas et al. 14 reported a prevalence of 47% among their 375 patients. They described a perifollicular distribution in some cases and occasionally scaling. The erythematous rash may be particularly accentuated on the antecubital fossa and axillary folds. 19 Skin biopsy shows some non‐specific features of viral‐related exanthema, such as slight spongiosis and mild perivascular lymphocytic infiltrate.
Vascular pattern
Several vascular lesions have been described in SARS‐CoV‐2 infection, including chilblain‐like lesions (the ‘COVID toe’), especially common in children, livedoid lesions, purpuric lesions and acral necrotic lesions. 20 Most of these clinical manifestations may have a thrombotic or microthrombotic pathological counterpart. In addition, cases of immune thrombocytopaenic purpura and of the antiphospholipid antibody syndrome have been described with their well‐known cutaneous manifestations. 21 , 22
SARS‐CoV‐2 infects the host using the angiotensin‐converting enzyme 2 (ACE2) receptor, which is expressed in several tissues, including endothelial cells. 23 The spectrum of the above‐mentioned vascular lesions may be due to different possibly overlapping mechanisms, including a direct action of the virus on endothelial cells, 24 an indirect effect involving the triggering of immune or autoimmune reactions as in the case of the immune thrombocytopenic purpura, or to an exaggerated and uncontrolled host response accompanying the well‐known ‘cytokine storm’. Whatever the starting mechanism, the consequent microvascular dysfunction can lead to increased vasoconstriction and organ ischaemia, inflammation and a further pro‐coagulation state. Whether skin lesions such as chilblain‐like lesions correlate with involvement of internal organs remains to be defined.
Chilblain‐like
Chilblain‐like or perniosis‐like skin lesions present as erythematous‐oedematous manifestations affecting acral sites, mostly toe and soles, with possible bullous evolution. They are usually asymmetrical and mostly itchy and/or painful. 25 They typically affect young patients in the absence of systemic symptoms or associated with low‐grade disease severity. Notably, they lack a history of chilblains or Raynaud phenomenon. In the case series reported by Recalcati, the eruptions resolved after 2–4 weeks without any treatment. 26 Bouaziz et al. 27 described chilblain‐like lesions in two patients, one presenting also Raynaud's phenomenon. A 19% prevalence of chilblain‐like lesions was documented in the Spanish series. 14 Histology shows a superficial and deep angiocentric and eccrinotropic lymphocytic infiltrate with some papillary dermal oedema, vacuolar degeneration of the basal layer and lymphocytic exocytosis to the epidermis and acrosyringia. 14 A puzzling finding in patients with chilblain‐like lesions is the low percentage of positivity to PCR for SARS‐CoV‐2. In one study on children and adolescents, only one of 19 patients was tested positive. 28 In a similar study on mostly paediatric patients, none of the 19 tested patients resulted positive. 29 This could be explained by the likely lower sensitivity of PCR for SARS‐CoV‐2 in mild cases and in children, possibly because of a low viral load. 30 Otherwise, chilblain‐like lesions could represent a late event in the disease course and PCR could become negative when assessed.
Purpuric/petechial lesions
A wide spectrum of purpuric and petechial lesions has been described as possible manifestations associated with SARS‐CoV‐2 infection (Fig. 1). They appear at any time during the COVID‐19 course, and they are localized on the trunk, buttock, limbs, typically in adult patients and could be associated with symptoms such as a burning sensation. These lesions may cause difficulties with differential diagnosis. Indeed, in Thailand a COVID‐19‐related skin rash with petechiae was initially misdiagnosed as Dengue. 31 Jimenez‐Cauhe et al. 32 described a patient with erythemato‐purpuric, millimetric, coalescing macules in flexural regions. In this case, the rash appeared after 3 days and he had started a therapy with hydroxychloroquine and lopinavir/ritonavir.
Figure 1.
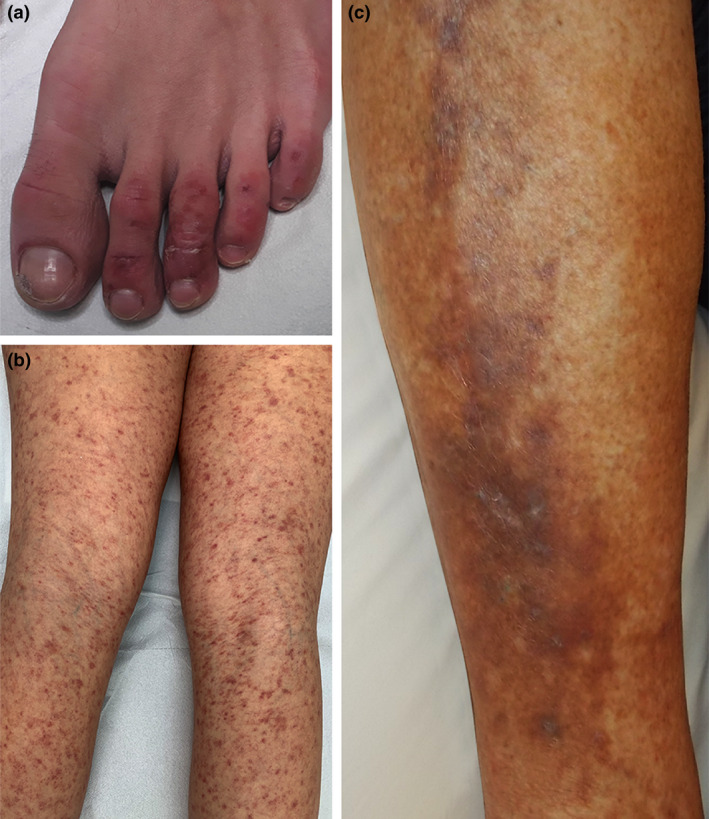
Vascular pattern of dermatological lesions associated with COVID‐19. (a) Chilblain‐like lesions on the toes; (b) purpuric lesions on the lower limbs; and (c) livedoid/purpuric plaques on the leg.
Magro et al. 33 described two patients with widespread purpuric skin manifestation. The first developed a retiform purpura with extensive surrounding inflammation on buttocks, while the second had a mildly purpuric reticulated eruptions on chest, legs and arms. Bouaziz et al. 27 described two patients with purpuric rash evolving in necrotic lesions and Zulfiqar et al. 34 reported a case of a lower‐extremity purpura as skin sign of immune thrombocytopenic purpura related to SARS‐CoV‐2 infection. Diaz‐Guimaraens et al. 35 observed a patient with erythematous macules, papules and petechiae affecting the popliteal fossae, buttocks and anterior thighs but spearing acral regions and mucosae.
Livedo
Livedoid/necrotic lesions appear any time during the disease course, and they are localized on the limbs, in older patients with more severe disease course. Manalo et al. 36 reported a patient who developed a transient non‐pruritic blanching unilateral livedoid patch on the right anterior thigh resembling livedo reticularis. Another patient showed a transient unilateral asymptomatic rash on her right leg resembling livedo reticularis that appeared after occasional sun exposure. It lasted almost 20 min and did not recur. According to the authors, the lesions could be explained by the underlying clotting disorders associated with COVID19. 36 Galván Casas et al. 14 considered livedo and necrosis as belonging to the same clinical pattern, suggesting a major systemic occlusive vascular disease. In their series, these lesions had a prevalence of 6% and were present in older patients and in patients with more severe disease.
Kawasaki disease
Very recently, an association between SARS‐CoV‐19 and Kawasaki syndrome has been reported, 37 , 38 an acute vasculitis of childhood, which can complicate with heart disease, mostly in developed countries. Such an association may corroborate the occurrence of an endothelial damage associated with SARS‐CoV‐19 infection.
Acro‐papular eruption
An acral papular eruption can be the expression of several viral diseases. Recently, papular eruptions with acral distribution have been associated with SARS‐CoV‐2 infection. Estébanez et al. 39 reported one case of COVID‐19 associated with the development of confluent erythematous‐yellowish pruritic papules 13 days after being tested for SARS‐CoV‐2. Despite topical steroid treatment, 3 days later, the lesions became confluent erythematous plaques with a pruritic component. Proposed immunopathological mechanisms for the viral associated papular eruptions include immune complexes or delayed hypersensitivity reactions, and it is plausible that similar immuno‐mediated mechanisms may be also operative in COVID‐19‐associated papular eruptions. 39
Urticaria‐like pattern
The urticarial‐like pattern has been described as a slightly pruritic disseminated erythematous skin rash that healed in few days. 40 In the study by Galván Casas et al., 14 urticarial lesions had a prevalence of 19%, appearing at the same time with systemic symptoms in the more severe COVID‐19 cases.
Discussion
Dermatologists could play a relevant role in response to the SARS‐CoV‐2 pandemic with the early recognition of skin lesions suggestive of COVID‐19. Skin manifestations may represent a relevant feature of COVID‐19, and these lesions may be under‐recognized because of the lack of routine dermatology consultations during the pandemic. Only patients with severe respiratory symptoms are usually screened for SARS‐CoV‐2 infection. As a result, it is quite difficult to accurately determine the actual prevalence of the infection. These considerations could explain the wide variations in the prevalence of cutaneous lesions reported in patients with SARS‐CoV‐2 infection ranging from 0.2% to 20.4%. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
Distinct cutaneous clinical patterns have been associated with COVID‐19. They appear at different times in the disease course and are associated with different duration, severity and prognosis. The different patterns usually do not coexist in the same patient, and patients who develop more than one pattern are very uncommon. 14
Among exanthema patterns, varicella‐like type is an early and quite specific skin manifestation of SARS‐CoV‐2 infection and could therefore represent a useful clue in asymptomatic or mild symptomatic patients. 13 It may be distinguished from a typical varicella exanthema since it is characterized by the presence of papules (which do not always evolve into vesicles) and histologically is not associated with a large intra‐epidermal blister, with only few acantholytic keratinocytes showing characteristic viropathic effects. Moreover, itching is only mild or absent. 13 A Tzanck test or varicella‐zoster PCR may be useful for the differential diagnosis. Interestingly, PCR for SARS‐CoV‐2 from vesicle may show negative results. 37 This may be related to the fact that PCR assays are not standardized for skin testing or the viral load is too low to be detected. Nonetheless, this finding suggests that varicella‐like vesicles in COVID‐19 patients are probably not contagious. Other than varicella, differential diagnoses may include dermatologic manifestations of herpes simplex, erythema multiforme, autoimmune bullous diseases such as dermatitis herpetiformis and bullous pemphigoid.
Urticarial and maculo‐papular exanthema might not be very helpful for diagnosis, as these are common and may have many different causes. Drug reactions may be an important and challenging differential diagnosis. Other differential diagnoses include acute febrile neutrophilic dermatosis (Sweet syndrome), acute urticaria and erythema multiforme.
Among vascular patterns, pseudo‐chilblain may look like perniosis, and as they appear late in the disease progression and are less commonly associated with virologic confirmation, it is possible that they are not immediately related with COVID‐19. 25 The differential diagnoses include local trauma, Raynaud phenomenon, acrocyanosis, erythromelalgia and purple toe syndrome associated with oral anticoagulant exposure. Livedoid and purpuric lesions are relatively uncommon and mostly appear in elderly and severe patients with COVID‐19. These might be primary lesions of SARS‐CoV‐2 infection or may reflect vascular occlusion complications, since COVID‐19 has been linked with alterations in coagulation and consequent vascular damage. Necrotic lesions appear late in the COVID‐19 patient and are therefore unlikely helpful for diagnosis. Differential diagnosis of livedoid and purpuric lesions includes all forms of livedoid vasculopathy, vasculitis, antiphospholipid syndrome, essential mixed cryoglobulinaemia, disseminated intravascular coagulation and idiopathic thrombocytopenic purpura.
An endothelial cell involvement in COVID‐19 patients was recently observed. 41 These cells express ACE2 receptors, through which SARS‐CoV‐2 manages to infect the host and the presence of viral elements, together with inflammatory cells, within them was histologically demonstrated. These findings suggest that SARS‐CoV‐2 infection may produce an endotheliitis in different organs, including the skin, as a direct effect of viral presence and of the host inflammatory response.
Papular eruption may simulate lichenoid dermatitis, or Gianotti–Crosti syndrome, a benign papular acrodermatitis that occurs in children, mostly associated with hepatitis B virus and Epstein–Barr virus infection and, less frequently, with other viruses and vaccination. 42
Overall, pseudo‐chilblain and vesicular lesions may represent the most common and characteristic skin manifestations of COVID‐19. However, since pseudo‐chilblain lesions more commonly appear later during the disease course and are not associated with severe disease, they might be more useful as epidemiological markers than for diagnosis. 43 , 44
Many issues should be addressed including the dynamic of viral load and its relation with the skin rash. The rash may represent an important clue to determine the optimal timing (before, during or after the skin rash) to collect the samples for molecular identification.
An important question is whether the dermatological manifestations in COVID‐19 may have any impact on the prognosis and consequent treatment of the disease. In this regard, it is unclear whether the cutaneous manifestations of SARS‐CoV‐2 infection are the result of an aberrant immune response and consequently could predispose to systemic immune mediated diseases. 43 One may argue that the immunological response may sustain, not only an acute inflammatory reaction, but also the development of a late/chronic phase of an autoimmune postinfection process characterized by the absence of the infectious agent. In this case, the mechanisms which might be operative may include molecular mimicry and/or epitope spreading and the induction of an autoimmune process related to concomitant host and environmental factors. 45
Lastly, other skin manifestations to be considered are the adverse drug reactions to the drugs prescribed for the treatment of COVID‐19 including hydroxychloroquine (Fig. 2). Whether SARS‐CoV‐2 infection can directly cause a worsening of pre‐existing chronic inflammatory diseases such as psoriasis or atopic dermatitis remains to be determined.
Figure 2.
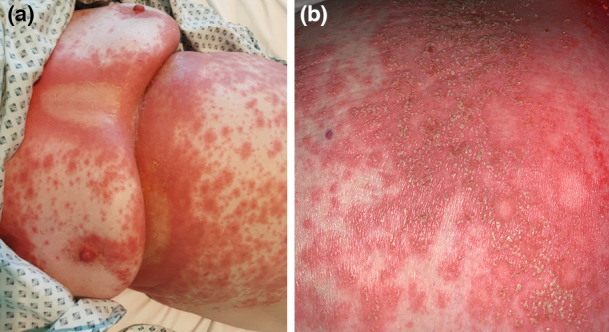
(a) Diffuse acute generalized exanthematous pustulosis as adverse drug reaction to hydroxychloroquine; and (b) details of pustules.
Dermatology's outlook in the COVID‐19 pandemic is multidimensional. It is important to make clinicians aware of the spectrum of dermatological manifestations of SARS‐CoV‐2 infection, improving viral testing and clinical management.
Acknowledgement
The patients in this manuscript have given written informed consent to publication of their case details.
Conflicts of interest
No conflict of interest to be declared for any authors.
Funding sources
None declared.
References
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55: 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su CJ, Lee CH. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol 2020; 15: e251–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID‐19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol 2020; 24. 10.1111/jdv.16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mungmungpuntipantip R, Wiwanitkit V. COVID‐19 and cutaneous manifestations. J Eur Acad Dermatol Venereol 2020; 34. 10.1111/jdv.16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson Regnault M. Comment on “Cutaneous manifestations in COVID‐19: a first perspective” by Recalcati S. J Eur Acad Dermatol Venereol 2020; 21. 10.1111/jdv.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez‐Nieto D, Ortega‐Quijano D, Segurado‐Miravalles G, Pindado‐Ortega C, Prieto‐Barrios M, Jimenez‐Cauhe J. Comment on: cutaneous manifestations in COVID‐ 19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol 2020; 34. 10.1111/jdv.16470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avellana Moreno R, Estela Villa LM, Avellana Moreno V, Estela Villa C, Moreno Aparicio MA, Avellana Fontanella JA. Cutaneous manifestation of COVID‐19 in images: a case report. J Eur Acad Dermatol Venereol 2020; 19. 10.1111/jdv.16531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guarneri C, Venanzi Rullo E, Gallizzi R, Ceccarelli M, Cannavò SP, Nunnari G. Diversity of clinical appearance of cutaneous manifestations in the course of COVID‐19. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duong TA, Velter C, Rybojad M et al. Did Whatsapp® reveal a new cutaneous COVID‐19 manifestation? J Eur Acad Dermatol Venereol 2020; 24. 10.1111/jdv.16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skayem C, Cassius C, Ben Kahla M et al. Teledermatology for COVID‐19 cutaneous lesions: substitute or supplement? J Eur Acad Dermatol Venereol 2020; 18. 10.1111/jdv.16630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marzano AV, Genovese G, Fabbrocini G et al. Varicella‐ like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83: 280–285. 10.1016/j.jaad.2020.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galván Casas C, Català A, Carretero Hernández G. Classification of the cutaneous manifestations of COVID‐ 19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 29. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahouach B, Harant S, Ullmer A. Cutaneous lesions in a patient with COVID‐19: are they related? Br J Dermatol 2020; 30. 10.1111/bjd.19168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrero‐Moyano M, Capusan TM, Andreu‐Barasoain M et al. A clinicopathological study of 8 patients with COVID‐19 pneumonia and a late‐onset exanthema. J Eur Acad Dermatol Venereol 2020; 19. 10.1111/jdv.16631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skroza N, Bernardini N, Balduzzi V et al. A late onset widespread skin rash in a previous Covid‐19 infected patient: viral or multidrug effect? J Eur Acad Dermatol Venereol 2020; 18. 10.1111/jdv.16633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reymundo A, Fernáldez‐Bernáldez A, Reolid A et al. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients. J Eur Acad Dermatol Venereol 2020; 4. 10.1111/jdv.16707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with Coronavirus Disease 2019? J Eur Acad Dermatol Venereol 2020; 34: 246–247. 10.1111/jdv.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dominguez‐Santas M, Diaz‐Guimaraens B, Garcia Abellas P et al. Cutaneous small‐vessel vasculitis associated with novel 2019 coronavirus SARS‐CoV‐2 infection (COVID‐19). J Eur Acad Dermatol Venereol 2020; 26. 10.1111/jdv.16663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García‐Gil MF, García García M, Monte Serrano J, Prieto‐Torres L, Ara‐Martín M. Acral purpuric lesions (Erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol 2020; 20. 10.1111/jdv.16644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Xiao M, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020; 382: 382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monteil V, Kwon H, Prado P. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell 2020; 181: 905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; 24. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Recalcati S, Barbagallo T, Frasin LA. Acral cutaneous lesions in the Time of COVID‐19. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouaziz JD, Duong T, Jachiet M. Vascular skin symptoms in COVID‐ 19: a french observational study. J Eur Acad Dermatol Venereol 2020; 27. 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andina D, Noguera‐Morel L, Bascuas‐Arribas M et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol 2020; 37: 406–411. 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. López‐Robles J, de la Hera I, Pardo J, Martínez J, Cutillas‐Marco E. Chilblain‐like lesions: a case series of 41 patients during the COVID‐19 pandemic. Clin Exp Dermatol. 2020; 5. 10.1111/ced.14275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tagarro A, Epalza C, Santos M et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr 2020; 8: e201346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020; 82: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez‐Cauhe J, Ortega‐Quijano D, Prieto‐Barrios M, Moreno‐Arrones OM, Fernandez‐Nieto D. Reply to “COVID‐19 can present with a rash and be mistaken for Dengue”: petechial rash in a patient with COVID‐19 infection. J Am Acad Dermatol 2020; 10: S0190‐9622(20)30556‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magro C, Mulvey JJ, Berlin D et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res 2020; 5244: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zulfiqar AA, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid‐19. N Engl J Med 2020; 382: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diaz‐Guimaraens B, Dominguez‐Santas M, Suarez‐Valle A et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020; 30. 10.1001/jamadermatol.2020.1741 [DOI] [PubMed] [Google Scholar]
- 36. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient Livedo Reticularis. J Am Acad Dermatol 2020; 10. 10.1016/j.jaad.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandez‐Nieto D, Ortega‐Quijano D, Jimenez‐Cauhe J et al. Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol 2020.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones VG, Mills M, Suarez D. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatrì 2020; 10: 537–540. [DOI] [PubMed] [Google Scholar]
- 39. Estébanez A, Pérez‐Santiago L, Silva E, Guillen‐Climent S, García‐Vázquez A, Ramón MD. Cutaneous manifestations in COVID‐ 19: a new contribution. J Eur Acad Dermatol Venereol 2020; 34: 250–251. 10.1111/jdv.16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: 244–245. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leung AKC, Sergi CM, Lam JM, Leong KF. Gianotti‐Crosti syndrome (papular acrodermatitis of childhood) in the era of a viral recrudescence and vaccine opposition. World J Pediatr 2019; 15: 521–527. [DOI] [PubMed] [Google Scholar]
- 43. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 Pandemic. Int J Dermatol 2020; 59: 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: a diagnostic clue. Pediatr Dermatol 2020; 37: 435–436. 10.1111/pde.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suchonwanit P, Leerunyakul K, Kositkuljorn C. Cutaneous manifestations in COVID‐19: lessons learned from current evidence. J Am Acad Dermatol 2020; 83: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


