1. INTRODUCTION
The detection of SARS‐CoV‐2 in the saliva of patients with coronavirus disease (COVID‐19) has made this biological fluid relevant in terms of the diagnosis and transmission of the infection (Azzi, Carcano, Gianfagna, et al., 2020; To, Tsang, Yip, et al., 2020). As a result, the dental clinic is considered an environment of risk for dental healthcare personnel and their patients, particularly due to the potential transmission of the virus through droplets and aerosols (Peng et al., 2020). Based on this argument, it has been suggested that the measures for controlling cross‐infection during dental practice should include a preprocedural mouth rinse containing oxidative agents such as 1% hydrogen peroxide and 0.2% povidone iodine (PVP‐I) (Peng et al., 2020). This protocol, with small variations, has been accepted by the main professional dental associations worldwide, such as the American Dental Association (ADA, 2020). If an antiseptic mouthwash's virucidal activity is demonstrated, why limit its application to the dental clinic setting and not administer it routinely to the entire population to prevent the community transmission of this new coronavirus? To date, the efficacy of povidone against SARS‐CoV‐2 has not been confirmed. In this study, we analyzed the impact of a mouthwash with PVP‐I on the salivary viral load of SARS‐CoV‐2 in 4 patients with COVID‐19.
2. METHODS
2.1. Patient 1
A 74‐year‐old man with a history of B‐cell non‐Hodgkin's lymphoma was admitted to the hospital 28 days ago due to pneumonia by SARS‐CoV‐2. The patient underwent treatment with hydroxychloroquine, lopinavir + ritonavir, corticosteroids, and tocilizumab. The patient required admission to the intensive care unit and endotracheal intubation. The patient is currently hospitalized in the hospital ward, without exogenous oxygen supply, is undergoing treatment with rituximab, and presents positive serial polymerase chain reaction (PCR) tests of nasopharyngeal exudate.
2.2. Patient 2
A 73‐year‐old man who lived in a nursing home presented an initially positive PCR to SARS‐CoV‐2 in nasopharyngeal exudate 41 days ago. The patient has a history of diabetes and ischemic stroke and has an implanted dual‐chamber, rate‐modulated pacemaker. The patient had no symptoms suggestive of COVID‐19 but was hospitalized for sepsis by Escherichia coli secondary to a urinary tract infection.
2.3. Patient 3
A 43‐year‐old woman debuted with headaches, fever, cough, fatigue when walking, loss of taste, and sense of smell. The patient had no relevant medical history. The patient had an initially positive PCR to SARS‐CoV‐2 37 days ago and since then positive serial PCR tests in nasopharyngeal exudate. She therefore remained isolated at home.
2.4. Patient 4
A 54‐year‐old woman who worked in a nursing home debuted with dry cough and febricula. The patient had a history of spitzoid melanoma, arterial hypertension, and fibromyalgia. The PCR for SARS‐CoV‐2 was positive for the first time 37 days ago and remained positive thereafter.
Nasopharyngeal swabs were performed on all patients, and a baseline saliva sample was taken first thing in the morning, as previously described (To, Tsang, Yip, et al., 2020). The patients then performed a rinse with 15 ml of 1% povidone iodine for 1 min. Serial saliva samples were then taken at 5 min, 1 hr, 2 hr, and 3 hr after the rinse.
We performed nucleic acid extraction in a MicrolabStarlet IVD platform using the STARMag 96 × 4 Universal Cartridge Kit (Seegene). To detect SARS‐CoV‐2, we applied the Allplex™ 2019‐nCoV Assay (Seegene), a multiplex one‐step real‐time reverse transcription (rRT)‐PCR assay targeting a conserved region in the structural protein envelope E‐gene for pan‐Sarbecovirus detection, RNA‐dependent RNA polymerase (RdRP), and nucleocapsid (N) genes specific for SARS‐CoV‐2. For rRT‐PCR, we employed the CFX96™ system (Bio‐Rad Laboratories). We analyzed the results using Seegene Viewer‐specific 2019‐nCoV software (Seegene). To establish a linear regression curve and obtain the concentration in copies/ml (inversely related to the cycle threshold value), we used a standard EDX SARS‐CoV‐2 (Bio‐Rad Laboratories) that contains synthetic RNA transcripts of 5 genetic targets of SARS‐CoV‐2 (genes E, N, ORF1ab, RdRp, and S) at a known concentration.
The study was approved by the Institutional Review Board of the University Hospital of Vigo (CHUVI), Sergas, Vigo, Spain.
3. RESULTS
The presence of SARS‐CoV‐2 was confirmed in all of the patients’ baseline saliva samples; however, the PCR of the nasopharyngeal exudate was negative for patients 3 and 4. Table 1 lists the cycle threshold values corresponding to genes E, RdRP, and N obtained during the first 3 hr after rinsing with PVP‐I. In 2 of the 4 participants (patients 3 and 4), the PVP‐I resulted in a significant drop in viral load, which remained for at least 3 hr (Figure 1).
Table 1.
SARS‐CoV‐2 RNA in the nasopharyngeal swab and saliva samples as measured by the cycle threshold (Ct), at baseline and after performing a povidone iodine mouthwash
| Nasopharyngeal swab | Saliva samples | ||||||
|---|---|---|---|---|---|---|---|
| Basal | Basal | 5 min. P‐M | 1 hr P‐M | 2 hr P‐M | 3 hr P‐M | ||
| Patient 1 | Ct E | 19.82 | ‐ | 32.58 | 34.98 | 33.78 | 31.77 |
| Ct RdRp | 21.97 | ‐ | 33.74 | 36.50 | 38.72 | 33.26 | |
| Ct N | 23.17 | 35.63 | 35.25 | 35.63 | 35.73 | 34.56 | |
| Patient 2 | Ct E | 35.84 | 22.02 | 23.74 | 33.32 | 38.35 | 38.94 |
| Ct RdRp | 37.91 | 27.70 | 28.78 | 37.68 | ‐ | ‐ | |
| Ct N | 37.06 | 26.11 | 27.70 | 37.04 | 39.15 | ‐ | |
| Patient 3 | Ct E | ‐ | 37.08 | 32.99 | 35.68 | 36.35 | 35.42 |
| Ct RdRp | ‐ | 38.06 | 34.60 | 37.05 | 37.76 | 37.37 | |
| Ct N | ‐ | 37.89 | 35.83 | 37.18 | 39.92 | 37.67 | |
| Patient 4 | Ct E | ‐ | 27.84 | 28.09 | 30.51 | 35.04 | ‐ |
| Ct RdRp | ‐ | 31.40 | 31.14 | ‐ | 38.87 | ‐ | |
| Ct N | ‐ | 31.18 | 30.84 | 34.37 | 35.04 | 37.62 | |
Abbreviations: E, structural protein envelope E‐gene; N, nucleocapsid gene; P‐M, postmouthwash; RdRP, RNA‐dependent RNA polymerase.
Figure 1.
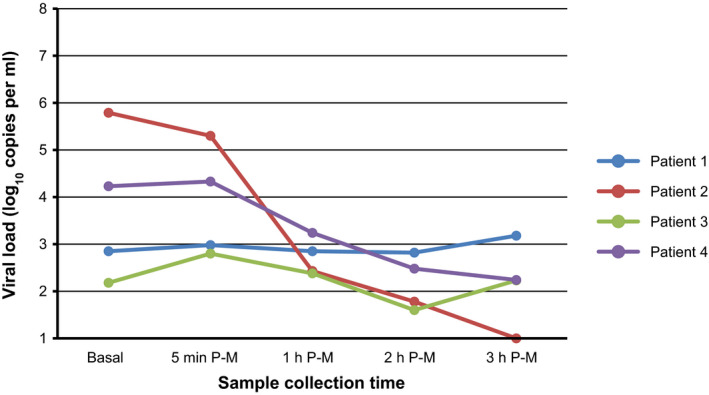
SARS‐CoV‐2 viral load in saliva samples at baseline and after performing a povidone iodine mouthwash. Basal: before mouthwash; P‐M: postmouthwash; min: minute; h: hour. For this figure, specimens with undetected viral load were assigned a value of 100 copies/ml
4. DISCUSSION
Coronaviruses are a group of single‐stranded RNA viruses that belong to the Nidovirales order and are classified into 4 genera (α, β, γ, and δ). The phylogenetic analysis of SARS‐CoV‐2 has shown that it belongs to the genus β‐CoV, as do severe acute respiratory syndrome coronavirus (SARS‐CoV) and the Middle East respiratory syndrome coronavirus (MERS‐CoV). In vitro studies have shown that PVP‐I has significant virucidal activity against SARS‐CoV and MERS, after a short period of exposure (Eggers, Koburger‐Janssen, Eickmann, & Zorn, 2018; Kariwa, Fuji, & Takashima, 2006). All β‐CoV have a similar structure, which is characterized by a lipid coating that constitutes the target of membrane‐disrupting agents such as PVP‐I (O’Donnell et al., 2020). It is therefore expected that SARS‐CoV‐2 would also be inactivated by PVP‐I, although the similarity of its nucleotide sequence to that of SARS‐CoV and MERS‐CoV is only 79% and 50%, respectively (Lu et al., 2020).
To date, the efficacy of povidone against SARS‐CoV‐2 has not been confirmed, as recognized by the ADA (ADA, 2020). This is probably one of the main reasons why the Centers for Disease Control have published the Interim Infection Prevention and Control Guidance for Dental Settings During the COVID‐19 Outbreak, which references the 2003 version indicating the insufficient evidence on the efficacy of preprocedural antimicrobial mouth rinses (Kohn et al., 2003).
The suitability of applying a classical antimicrobial such as PVP‐I would be established by studies that determine its ideal concentration and substantivity but especially that back its clinical efficacy. The limited number of patients enrolled in this study does not allow us to make adjustments for potential confounding factors such as viral load and the host's immune response. However, these preliminary in vivo results suggest that a PVP‐I rinse could reduce the saliva viral load of SARS‐CoV‐2 in patients with higher viral loads. Therefore, routine administration of PVP‐I would be primarily indicated for symptomatic patients infected with SARS‐CoV‐2, especially during the first week after symptom onset, which is when viral charges in the saliva are highest (To, Tsang, Leung, et al., 2020). Asymptomatic patients usually have low viral loads, but those who end up developing symptoms have substantially greater viral loads even during the presymptomatic phase (Zhou et al., 2020); accordingly, the application of PVP‐I for the general population could be considered as a supplementary prevention measure when a risk scenario is foreseen such as the generation of aerosols.
A casual finding in this study was the detection of viral RNA in saliva samples in 2 patients with negative PCR of nasopharyngeal exudate. We do not know if these patients are shedding live viruses or virions coated with host antibodies that render them non‐infectious. In any case, other researchers have achieved similar results, proposing that patients who have recovered should be discharged only after obtaining 2 negative throat samples and 1 negative saliva sample. If a saliva check is not performed, they recommend that, after being given a hospital discharge, the patient should remain in social isolation for least 14 days (Azzi, Carcano, Gasperina, et al., 2020).
In summary, given that a PVP‐I rinse is a simple, inexpensive, and practically innocuous intervention, we consider that the encouraging results of the present study justify implementing a clinical trial to confirm its efficacy.
CONFLICTS OF INTEREST
None to declare.
AUTHOR CONTRIBUTION
Lucía Martínez‐Lamas: Formal analysis; Investigation; Methodology; Supervision; Writing‐review & editing. Pedro Diz Dios: Conceptualization; Formal analysis; Supervision; Writing‐original draft. Maria Teresa Pérez Rodríguez: Data curation; Investigation; Methodology; Validation; Writing‐review & editing. Victor Del Campo Pérez: Conceptualization; Data curation; Methodology; Validation; Writing‐review & editing. Jorge Julio Cabrera Alvargonzalez: Data curation; Methodology; Software; Validation; Writing‐review & editing. Ana María López Domínguez: Data curation; Investigation; Methodology; Validation; Writing‐review & editing. Javier Fernández Feijoo: Funding acquisition; Project administration; Validation; Writing‐review & editing. Marcio Diniz‐Freitas: Data curation; Methodology; Validation; Writing‐review & editing. Jacobo Limeres Posse: Data curation; Formal analysis; Methodology; Supervision; Writing‐review & editing.
Martínez Lamas L, Diz Dios P, Pérez Rodríguez MT, et al. Is povidone iodine mouthwash effective against SARS‐CoV‐2? First in vivo tests. Oral Dis.2022;28(Suppl. 1):908–911. 10.1111/odi.13526
REFERENCES
- ADA Interim Guidance for Minimizing Risk of COVID‐19 Transmission. https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf. Accessed May 10, 2020
- Azzi, L. , Carcano, G. , Gasperina, D. D. , Sessa, F. , Maurino, V. , & Baj, A. (2020). Two cases of COVID‐19 with positive salivary and negative pharyngeal or respiratory swabs at hospital discharge: A rising concern. Oral Diseases, 1–3. 10.1111/odi.13368. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi, L. , Carcano, G. , Gianfagna, F. , Grossi, P. , Gasperina, D. D. , Genoni, A. , … Baj, A. (2020). Saliva is a reliable tool to detect SARS‐CoV‐2. Journal of Infection, 81(1), e45–e50. 10.1016/j.jinf.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, M. , Koburger‐Janssen, T. , Eickmann, M. , & Zorn, J. (2018). In vitro bactericidal and virucidal efficacy of povidone‐iodine gargle/mouthwash against respiratory and oral tract pathogens. Infectious Diseases and Therapy, 7, 249–259. 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa, H. , Fuji, N. , & Takashima, I. (2006). Inactivation of SARS coronavirus by means of povidone‐iodine, physical conditions and chemical reagents. Dermatology (Basel), 212(Suppl 1), 119–123. 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, W. G. , Collins, A. S. , Cleveland, J. L. , Harte, J. A. , Eklund, K. J. , & Malvitz, D. M. & Centers for Disease Control and Prevention (CDC) . (2003). Guidelines for infection control in dental health‐care settings–2003. MMWR ‐ Recommendations and Reports, 52(RR‐17), 1–61. [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395, 565–574. 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell, V. B. , Thomas, D. , Stanton, R. , Maillard, J.‐T. , Murphy, R. C. , Jones, S. A. , Sattar, S. S. (2020). Potential role of oral rinses targeting the viral lipid envelope in SARS‐CoV‐2 infection. Function, 1(1), zqaa002, 10.1093/function/zqaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X. , Xu, X. , Li, Y. , Cheng, L. , Zhou, X. , & Ren, B. (2020). Transmission routes of 2019‐nCoV and controls in dental practice. International Journal of Oral Science, 12, 9. 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. , Tsang, O. T. , Leung, W. S. , Tam, A. R. , Wu, T. C. , Lung, D. C. , … Yuen, K. Y. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: An observational cohort study. The Lancet Infectious Diseases, 20, 565–574. 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. , Tsang, O. T. , Yip, C. C. , Chan, K. H. , Wu, T. C. , Chan, J. M. C. , Yuen, K. Y. (2020). Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases, 1–3. 10.1093/cid/ciaa149. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, R. , Li, F. , Chen, F. , Liu, H. , Zheng, J. , Lei, C. , & Wu, X. (2020). Viral dynamics in asymptomatic patients with COVID‐19. International Journal of Infectious Diseases, 96, 288–290. 10.1016/j.ijid.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


