1.
>To the Editor,
“Misfortune might be a blessing in disguise.” – Tao‐Te‐Ching (Book of the Way) by Lao‐Tzu, 350 BCE
Since its first report in Wuhan, China, in December 2019, the novel pandemic COVID‐19, caused by SARS‐CoV‐2 virus, has rampaged throughout the world. 1 People with asthma and allergies are usually at greater risk of more severe outcomes with virus infections. However, recent reports have accumulated evidences that the prevalence of allergic diseases and asthma in patients with COVID‐19 is lower than expected among other comorbidities and risk factors of the severe form of COVID‐19 (Appendix S1 for additional reference 1‐3). Why are then allergic diseases and asthma underrepresented as co‐morbid risk factors in patients with COVID‐19? Is this a sampling bias in the currently published clinical reports or is there a real discrepancy in the prevalence of asthma among COVID‐19‐infected patients that may glean a light for us to fight this pandemic? Here, we hypothesize the plausible mechanisms in asthmatics based on available publications (Appendix S1) that may have effects in determining their susceptibility to and disease severity with SARS‐CoV‐2 infection.
Firstly, coronavirus recognition and infection are dependent on the cellular receptors, the angiotensin‐converting enzyme 2 (ACE2) for docking spike protein of SARS‐CoV‐2, and transmembrane protease serine 2 (TMPRSS2) to cleave the docked spike protein for virus entry by membrane fusion (Appendix S1, Reference 4, 5). The gene expression levels of ACE2 and TMPRSS2 are influenced by the genetic variants of the host and microbial infections, and induced by innate immune response, such as the production of interferons and mucins. 2 In asthma patients, the expression of these two molecules in the respiratory epithelial cells during SARS‐CoV‐2 infection is determined by the age, gender, comorbidity, and type 2 allergic inflammation. Jackson et al 3 found asthma and respiratory allergy are associated with reduced ACE2 expression in airway cells based on their patient cohorts. In contrast, Sajuthi et al 4 demonstrated that TMPRSS2 is part of a mucus secretory network, highly upregulated by type 2 allergic inflammation mainly by interleukin‐13. Peters et al 5 found the gene expression for ACE2 and TMPRSS2 in the cells obtained from induced sputum did not differ between healthy subjects and asthmatics. Therefore, these reports did not reveal convincing information, regarding whether asthmatic patients have lower expression of ACE2 and serine protease TMPRSS2 for SARS‐CoV‐2 based on their allergic status and/or chronic lung inflammation. Particularly, these studies lack the effect of SARS‐CoV‐2‐specific analysis and observations in asthmatic patients to be able to reach a conclusion in the real world.
Secondly, viral load and immune response of the host determine the final outcome and/or the severity of ARDS and multiple organ failure in COVID‐19‐infected patients. For asthmatic patients, the innate immune response to COVID‐19 infection may be impaired due to lower levels of IFNγ in their bronchial epithelial cells, but it may also be favourable in reducing ACE2 expression, which is depended on IFNγ production. 4 In addition to IFNs, there are other molecules of innate immunity in the respiratory tract that may also have anti‐viral functions, such as mannose‐binding lectin (MBL), and surfactant protein A (SP‐A) and D (SP‐D) that are produced by alveolar type 2 cells in the lung, which are also largely infected by SARS coronavirus. These molecules, MBL and SP‐D, found in higher concentrations in the BALF of patients with asthma and respiratory allergy and are increased due to chronic inflammation, have been identified to bind spike protein of SARS coronavirus, inhibit its binding of the ACE2 cellular receptor and are thereby able to protect the alveolar macrophages from virus‐induced activation 6 , 7 Recently, trained immunity found in the myeloid cells and alveolar macrophages of asthmatics may provide anti‐viral immunity in specific organs such as the lungs (Appendix S1, reference 6‐8). Although this hypothesis has not been validated in patients with COVID‐19, clinical trials to boost trained innate immunity by BCG vaccination to protect against COVID‐19 have been initiated in several countries. 8
Finally, we highlight that therapeutic medications and biologics used for asthma control may have some beneficial pharmacological effects in COVID‐19 infections. From in vitro models, inhaled corticosteroids alone or in combination with bronchodilators have been shown to suppress coronavirus replication and cytokine production (Appendix S1, reference 9). Asthmatic patients using inhaled corticosteroids (ICS) demonstrated lower expression of ACE2 and TMPRSS2 in their bronchial epithelial cells. 5 There is a clinical report of the improvement in three COVID‐19‐infected patients after using inhaled ciclesonide, although this study did not have proper controls (Appendix S1, reference 10). Clinical observation in children with severe asthma who received anti‐IgE monoclonal antibody (omalizumab) have shown decreased duration of human rhinovirus infections, viral shedding and risk of virus‐related exacerbation (Appendix S1, reference 11). In vitro, omalizumab attenuated plasmacytoid dendritic cell (pDC) FcεRIα protein expression while simultaneously augmenting pDC IFN‐α responses to rhinovirus and influenza virus (Appendix S1, reference 12). Together, these findings provide direct evidence that blocking IgE decreases susceptibility to respiratory viral illnesses through enhanced IFN‐α responses in pDCs. More interestingly, azithromycin combined with hydroxychloroquine in an open‐label nonrandomized clinical trial for COVID‐19‐infected patients has significantly decreased the viral load after six days of treatment compared with untreated controls (Appendix S1, reference 13). Azithromycin has been demonstrated to reduce the frequency of asthma exacerbation and improve the quality of life of asthmatic adults and preschool children with asthma that was not adequately controlled on standard inhaler therapy (Appendix S1, reference 14, 15). Although the mechanisms of this anti‐inflammatory effect in asthma is still not well defined, Gielen and co‐workers (Appendix S1, reference 16) demonstrated that azithromycin augments IFN‐β and IFN‐λ production and decreasing rhinovirus replication and release from rhinovirus‐infected human bronchial epithelial cells in vitro. Other studies also presented reduction levels of IL‐6 and TNF‐α after azithromycin treatment (Appendix S1, reference 17, 18). More interestingly, it is suggested that azithromycin may have protective effects in reducing SARS‐CoV‐2 invasion by interfering with ligand/CD147 receptor interactions, a novel SARS‐CoV‐2 cellular receptors beside ACE2, and decreasing the expression of some metalloproteinases (downstream to CD147) in primary human bronchial epithelial infected with rhinovirus (Appendix S1, reference 19). However, controlled clinical trials using azithromycin to treat patients with COVID‐19 (not involving asthmatic subjects) are now registered in several countries with results still pending.
In summary, we have seen a new zoonotic coronavirus, SARS‐CoV‐2, infection that has had a devastating effect on the host immunity via the inhibition of interferons leading to aberrant innate immune response, macrophage inflammation in releasing a cytokine storm and exhaustion of the cellular immunity of T lymphocytes. 9 Fortunately, due to chronic and sustained type 2 immune inflammation in the lungs of asthmatic patients, or by the medications they use for asthma control, it seems asthma may not be a major confounding disease in COVID‐19 infection, and this unexpected phenomenon may shed a new light on finding therapies or preventative strategies for SARS‐CoV‐2 (Figure 1). We still need a more comprehensive and in‐depth immune analysis of SARS‐CoV‐2 infection in the coming days to explore this hypothesis. However, all standard asthma therapies, whether inhaled steroids, combination of inhaled steroid plus long acting bronchodilator therapies or monoclonal antibodies like omalizumab and azithromycin, should be continued to be used to optimize asthma controls. For they can not only substantially reduce the risk of asthma exacerbation, but also will reduce risks and severe outcomes of COVID‐19.
Figure 1.
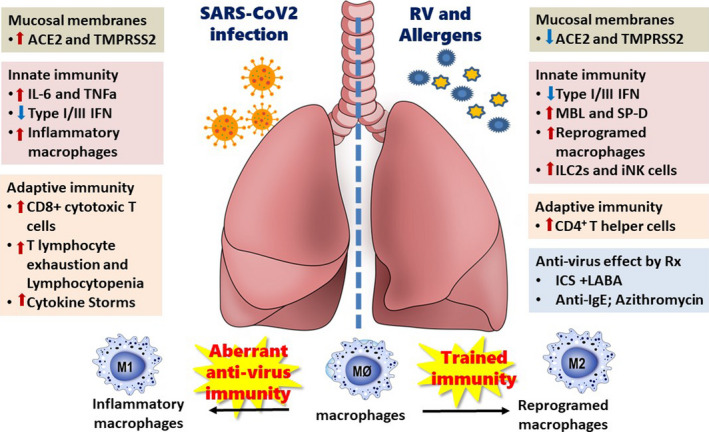
The immunopathogenesis of COVID‐19 and asthma
CONFLICT OF INTEREST
All authors declare no conflict of interests.
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016‐1035.e19. 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma and expression of the SARS‐CoV‐2 receptor, ACE2. J Allergy Clin Immunol. 2020;146(1):203‐206.e3. 10.1016/j.jaci.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sajuthi SP, DeFord P, Jackson ND, Montgomery MT, Everman JL, Rios CL, et al. Type 2 and interferon inflammation strongly regulate SARS‐ CoV‐2 related gene expression in the airway epithelium. Preprint at bioRxiv, 2020. 10.1101/2020.04.09.034454 [DOI] [PMC free article] [PubMed]
- 5. Peters MC, Sajuthi S, Deford P, et al. COVID‐19 related genes in sputum cells in asthma: Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83‐90. 10.1164/rccm.202003-0821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leth‐Larsen R, Zhong F, Chow VT, Holmskov U, Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology 2007;212:201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Lu K, Pfefferle S, et al. A single asparagine‐linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose‐binding lectin through multiple mechanisms. J Virol 2010;84:8753‐8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Neill LAJ, Netea MG. BCG‐induced trained immunity: can it offer protection against COVID‐19? Nat Rev Immunol. 2020;20(6):335‐337. 10.1038/s41577-020-0337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52(6):910‐941. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
JYW is supported by the Center for Allergy and Clinical Immunology Research (ACIR), Research and Service Headquarter, and in part by the Headquarters of University Advancement, National Cheng Kung University, Tainan, Taiwan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


