Abstract
Introduction
We describe findings from a large study that provide empirical support for the emerging construct of cognitive frailty and put forth a theoretical framework that may advance the future study of complex aging conditions. While cognitive impairment and physical frailty have long been studied as separate constructs, recent studies suggest they share common etiologies. We aimed to create a population predictive model to gain an understanding of the underlying biological mechanisms for the relationship between physical frailty and cognitive impairment.
Methods
Data were obtained from the longitudinal “Invecchaiare in Chianti” (Aging in Chianti, InCHIANTI Study) with a representative sample (n = 1453) of older adults from two small towns in Tuscany, Italy. Our previous work informed the candidate 132 single nucleotide polymorphisms (SNPs) and 155 protein biomarkers we tested in association with clinical outcomes using a tree boosting, machine learning (ML) technique for supervised learning analysis.
Results
We developed two highly accurate predictive models, with a Model I area under the curve (AUC) of 0.88 (95% confidence interval [CI] 0.83‐0.90) and a Model II AUC of 0.86 (95% CI 0.80‐0.90). These models indicate cognitive frailty is driven by dysregulation across multiple cellular processes including genetic alterations, nutrient and lipid metabolism, and elevated levels of circulating pro‐inflammatory proteins.
Discussion
While our results establish a foundation for understanding the underlying biological mechanisms for the relationship between cognitive decline and physical frailty, further examination of the molecular pathways associated with our predictive biomarkers is warranted. Our framework is in alignment with other proposed biological underpinnings of Alzheimer's disease such as genetic alterations, immune system dysfunction, and neuroinflammation.
Keywords: bioinformatics, cognitive frailty, cognitive impairment, frailty, machine learning
1. INTRODUCTION
1.1. Objective
We present data from a large study that provide empirical support that there are shared clinical and biological mechanisms for cognitive impairment and physical frailty, termed “cognitive frailty.” 1 Our results suggest that a larger number of prognostic factors contribute to the heterogeneity seen in Alzheimer's disease (AD) than is currently recognized. Based on these study findings, we propose an updated hypothesis of multi‐system dysfunction that will help operationalize the emerging understanding of cognitive frailty and advance the future study of complex aging conditions.
1.2. Historical evolution and rationale
Associating cognitive impairment and physical frailty began approximately 20 years ago in studies of individuals with mild cognitive impairment (MCI) and AD. Prior to that time, few older adults with cognitive impairment were included in frailty studies. 2 The term “cognitive frailty” was first used in 2001 in relation to the clock drawing task as a measure for identifying individuals at high risk for AD. 3 Since 2001, definitions of cognitive frailty have evolved as shared features between physical frailty and cognitive impairment were identified and described. In 2013, the International Consensus Group from the International Academy of Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) examined this connection based on epidemiologic evidence and common etiologies suggesting that cognitive frailty may represent a precursor to neurodegenerative disorders. 1 The recommendations from IANA “Cognitive Frailty: Rationale and Definition” now provide a foundation from which to study the complex heterogeneity seen in AD. 1 Evidence of a common pathophysiology between the two conditions is mounting, including a shared brain AD pathology found in individuals with physical frailty. 4 , 6 , 7 , 8
RESEARCH IN CONTEXT
Systematic review: The study was informed by a previously published systematic review using traditional sources (e.g., PubMed) to investigate shared biological predictors for cognitive frailty. While there is shared pathophysiology, there have been few studies exploring the biological predictors for individuals presenting with cognitive frailty. These relevant citations are appropriately cited.
Interpretation: Our findings led to an updated hypothesis describing cognitive frailty as a result of multi‐system dysfunction. This hypothesis closely aligns with the aging theory of cellular senescence; the concept of biological cellular damage and aberrant gene expression.
Future directions: We propose a theoretical framework and two statistical models that will allow for the generation of future hypotheses, and that can guide the design of additional studies of complex aging. Future exploration can include: (a) patterns of biomarker change, disease progression, and directions and rates of change over time; (b) the potential pathways associated with significant genes and protein biomarkers; (c) the relationship between high risk medications and associated gene variants for drug metabolism; and (d) the role of cellular senescence in cognitive frailty.
Physical frailty and cognitive impairment are considered complex aging syndromes with pathology that often involves more than one physiological system. In studying complex aging syndromes, scientists have often focused on a small number of variables in association with one type of pathology or phenotype. Yet, a growing number of seemingly unrelated factors are now associated with dementia risk including: impaired sleep, depression, inflammation, toxicity exposure (eg, pesticides, medications), and vascular and genetic risk factors. 9 , 10 , 11 These risk factors collectively can cause a “deficit accumulation.” 9 Deficit accumulation occurs when the number of insults to the body surpasses the ability for the damage to be removed or repaired. 12 Therefore, research that accounts for multiple risk factors using system‐based approaches has the potential to greatly facilitate our understanding of complex aging syndromes. 8
Such considerations also inform our approach with the view that machine learning (ML) statistical approaches can model complex biological systems such as diseases of aging. One emerging analytical tool is the use of bioinformatics; the process of analyzing complex biological data such as genetic codes with ML predictive analytic methods. ML methods for studying large amounts of available clinical and biological (“big‐omics”) data can be hypothesis generating, expand our capacity to learn from data, and accelerate discovery and disease prediction with the potential to impact clinical care. 13
In recent studies, our team has demonstrated ML methods to be effective in distinguishing patients with Parkinson's disease from healthy patients and identifying individuals with prodromal or preclinical Parkinson's disease. 12 We now seek to apply these methods to understanding the clinical and biological factors for individuals with cognitive frailty.
1.3. Updated hypothesis
Our study design was informed by findings from a previously published systematic review. In this review we collated results from previous studies on physical frailty and cognitive impairment and examined the overlap between predictive variables in these two historically independent conditions. 14 From a review of 342 studies, 456 protein and genetic single nucleotide polymorphisms (SNPs) were found to be predictive of both physical frailty and cognitive impairment. From this systematic review we identified overlapping cardiovascular risk factors (diabetes, dyslipidemia, hypertension); neuroinflammatory proteins (IL‐6, tumor necrosis factor [TNF]‐alpha, IL‐18, and IL‐1 beta) and their associated SNPs (IL6 rs1800796, TNF rs1800629, IL‐18 rs360722, and IL1‐beta rs16944); and multi‐system physiological changes such as nutritional, hematologic, renal, and hormonal biomarkers. 14 , 15 , 16 , 17 These findings informed the current study goal to explore multi‐system dysfunction as the underlying pathology for the association of physical frailty with cognitive impairment. Herein, we put forth a theoretical framework of complex systems for the future study of cognitive frailty (Figure 1).
FIGURE 1.
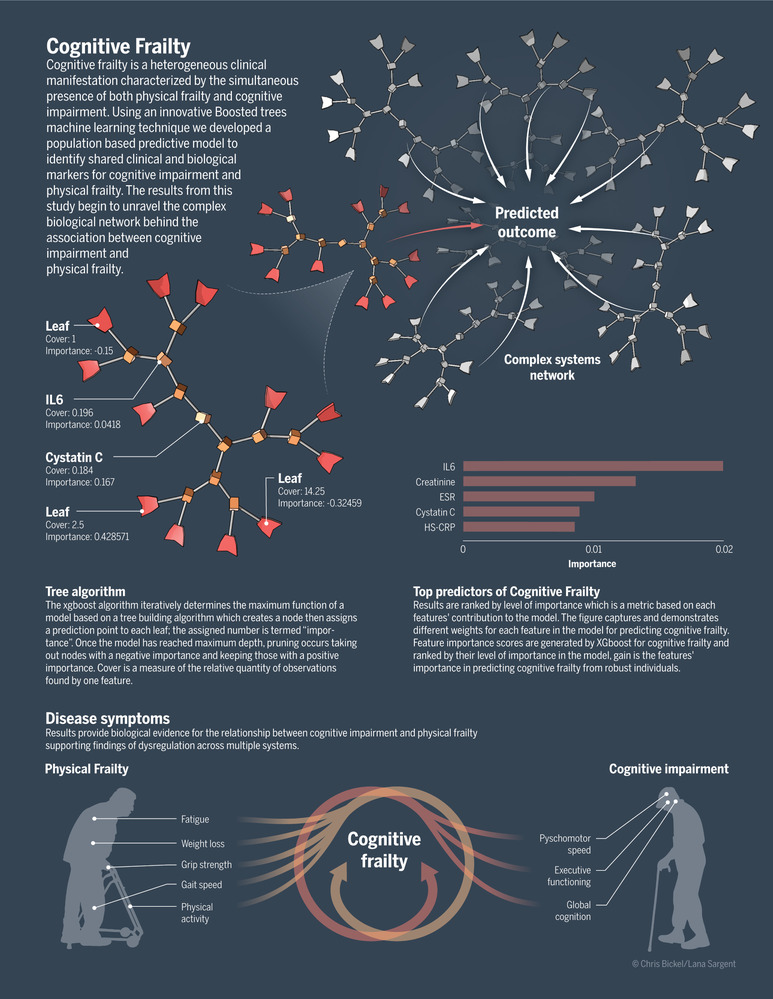
Theoretical framework for future study of cognitive frailty
2. METHODS
2.1. Study design
Participants were a part of the longitudinal study in aging Invecchiare in Chianti. All human subjects provided informed consent and all sample and data collection was approved by the ethics committee at Centre de recherché Clinique du CHUS. Two statistical predictive models were built to evaluate genetic variants (eg, SNPs), protein expression, and clinical markers as predictors of cognitive frailty. Model I tested prediction of genetic, protein, and clinical markers of cognitive frailty using criteria from the Mini‐Mental State Examination (MMSE), while Model II tested prediction of genetic, protein, and clinical markers of cognitive frailty with additional neuropsychological testing using the Trail Making Tests (TMT, Part A and B). 18 , 19 Three instruments were used to measure neuropsychological dimensions of cognitive frailty as determined by Delrieu et al. 20 The MMSE as a test of global mental status, the TMT‐A as a test of executive function, and the TMT‐B to assess attention. 21 TMT, part A and B cutoff scores are based on established norms for mild neurocognitive disorders, 19 and normative data for time to complete the TMT tests in seconds was stratified by age and education category. 19 The criteria of cognitive impairment was based on an MMSE score ≦23, a TMT‐A ≧78, and a score of ≧106 on the TMT‐B. 18 , 19 The InCHIANTI criteria for frailty is defined by Fried et al. as exhaustion, slowness, low physical activity, weakness, and weight loss. 2 Additional description of the InCHIANTI data collection and frailty classifications have been previously published. 22 , 23 Individuals with evidence of both physical frailty and cognitive impairment without a baseline clinical diagnosis of AD or other dementia were defined as having the cognitive frailty phenotype. 20 (See the Appendix in supporting information for study population and measurement details.) Based on our previously published systematic review, which identified shared biological markers for physical frailty and cognitive impairment, we tested 132 SNPs and 155 protein biomarker (total of 287) variables that were available in the InCHIANTI database in association with frailty and cognitive impairment (see the Appendix in supporting information for biomarker details). 14
2.2. Statistical approach
In this cross‐sectional study we used Extreme Gradient Boosting (xgboost) in R statistical software, to build a reproducible predictive model with large numbers of predictors. xgboost provides more efficient and accurate predictive modeling with large datasets and a rapid effective framework for feature selection. 24 The advantage of using a tree boosting approach model for the evaluation of multiple variables simultaneously is that it provides a high predictive value with low bias. 24 Boosted trees use individual decision trees that account for multi‐collinearity between variables and that retain only the best features in the final model. 24 Additionally, parameters are set to prevent overfitting of the models. The statistical analysis was completed in three steps: (1) analysis of all available variables for feature selection and data reduction; (2) model discovery followed by model validation; and (3) univariate analysis, t‐tests for continuous, and chi‐squared tests for binomial traits with a Bonferroni correction used to determine the significance of the variables between cognitive frailty and healthy older adults. To evaluate additive effects of the SNPs, a positive regression coefficient was used to indicate that each copy of the allele of interest increased the risk for cognitive frailty. 25
To evaluate the statistical models, we used the metric “area under the curve” (AUC). AUC was calculated from each model and used to determine discrimination of participants with cognitive frailty (cases) from healthy older adults (controls) in the training cohort. Covariates were selected to control for potential confounding effects, including sex, age, education, baseline diagnosis of dementia (n = 82), vascular dementia (n = 41), depression (n = 412), and Parkinson's disease (n = 16). (See the Appendix in supporting information for workflow and statistical analysis details.)
3. RESULTS
Of the 1453 adults participating, 1326 provided blood samples at study entry. (See Table 1 for sample characteristics of participants with cognitive frailty.) For discrimination of participants with cognitive frailty versus healthy older adults, the AUC of Model I was 0.88 (95% confidence interval [CI] 0.83–0.90) and 0.86 (95% CI 0.80–0.90) for Model II. We noted a normal distribution of AUCs across all iterations, with no statistically significant deviation from the expected values in any group, suggesting a good model fit. Both models showed high accuracy with AUCs ranging from 0.81–0.88 for Model I and 0.81–0.86 Model II.
TABLE 1.
Sample characteristics of participants with cognitive frailty for Model I and Model II
| Model I | P‐value | Model II | P‐value | |||
|---|---|---|---|---|---|---|
| Phenotype (n) | ||||||
| Control | 898 | 733 | ||||
| Cognitive Frailty | 257 | 412 | ||||
| Sex, (n) | Male | Female | Male | Female | ||
| Control | 418 | 480 | 372 | 372 | ||
| Cognitive frailty | 82 | 175 | 150 | 150 | ||
| Age, mean (SE) | ||||||
| Control | 73 (0.22) | <.0001 | 61 (.50) | <.0001 | ||
| Cognitive frailty | 82 (0.41) | 76 (.67) | ||||
| Anticholinergic Burden, mean (SE) | ||||||
| Control | 2.2 (0.10) | <.0001 | 1.9 (.08) | <.0001 | ||
| Cognitive frailty | 3.0 (0.21) | 3.0 (.21) | ||||
| Education, % | ≧High school | ≧High school | ||||
| Control | 6% | <.0001 | 10% | <.0001 | ||
| Cognitive frailty | 0 | 2% | ||||
Abbreviation: SE, standard error
The biomarkers identified as statistically significant between healthy older adults and individuals with cognitive frailty are discussed below. Biomarkers are ranked by level of importance based on its contribution to the model in Figures 2 and 3. (See Appendix Tables III–IV in supporting information for detail on significant biomarkers and association with specific cognitive domains.)
FIGURE 2.
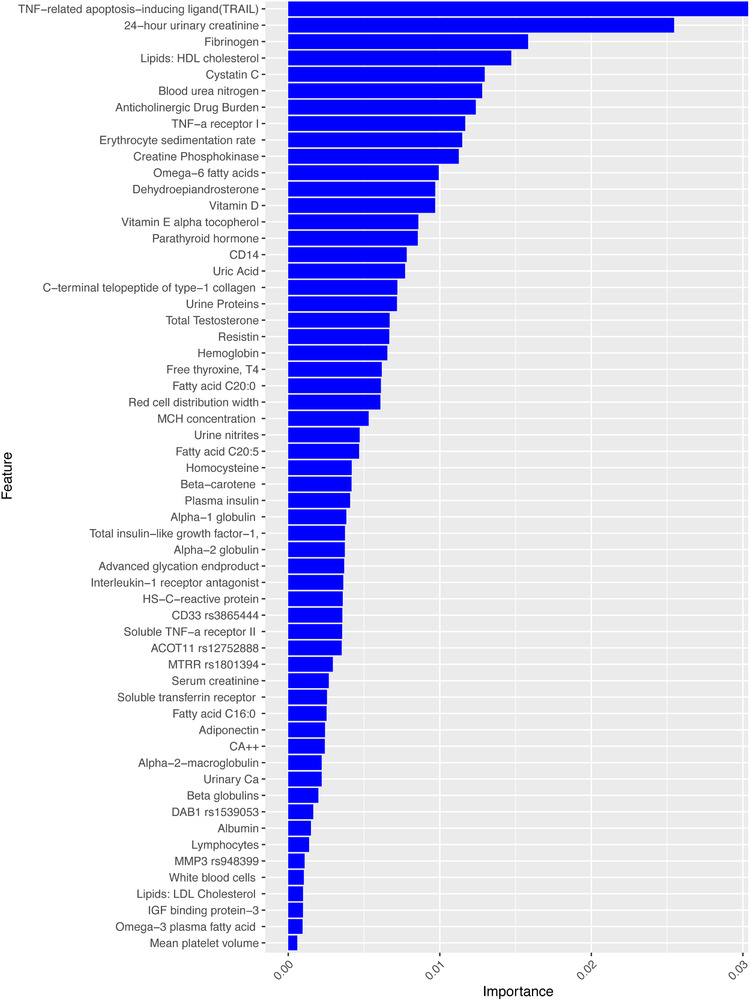
Feature importance scores for cognitive frailty in Model I. Note: Feature importance scores are generated by xgboost for cognitive frailty and ranked by their level of importance in the model. The figure demonstrates different weights for each feature's importance in predicting cognitive frailty from healthy individuals
FIGURE 3.
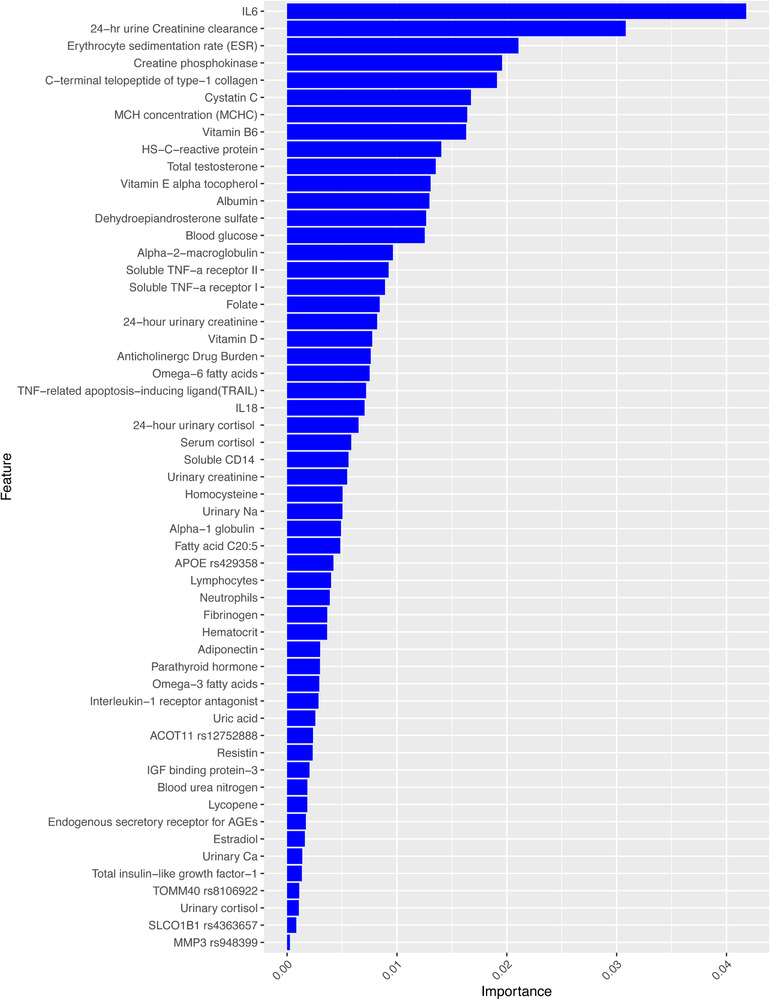
Feature importance scores for cognitive frailty in Model II. Note: Feature importance scores are generated by xgboost for cognitive frailty and ranked by their level of importance in the model. The figure demonstrates different weights for each feature's importance in predicting cognitive frailty from healthy individuals
3.1. Genomic predictors of cognitive frailty for Models I and II
Table 2 represents statistically significant gene polymorphisms (SNPs) for cognitive frailty. SNPs with significant differences between healthy older adults and cognitive frailty included: Model I (ACOT11) rs12752888 (P = .001), DAB1 rs1539053 (P = .01), (MMP3) rs948399 (P = .01), CD33 rs3865444 (P = .03), MTRR rs1801394 (P = .001), and Model II (ACOT11) rs12752888 allele C (TMT, P = .01), apolipoprotein E (APOE) rs429358 allele C (P = .01), SLCO1B1 rs4363657 allele C (P = .02), TOMM40 rs8106922 allele G ( P = .05), and (MMP3) rs948399 allele C (P = .05). In this study, genetic factors for cognitive frailty support functional genes that have been associated with AD and physical frailty. These include genes such as APOE allele C that is associated with executive function, TOMM40 associated with attention, rs12752888 allele C associated with all domains of cognitive decline, DAB1 associated with global cognition, and MTRR polymorphisms linked to two to four times greater odds of having physical frailty. 26 The level of importance and contribution of each SNP in predicting cognitive frailty can be evaluated amongst other biological markers in the statistical model (see Figures 2 and 3).
TABLE 2.
Genomic features for cognitive frailty Model I and Model II
| Model I | Gene | SNP‐associated allele | Chromosome | xgboost rank importance | β | SE | P‐value |
|---|---|---|---|---|---|---|---|
| CD33 | rs3865444_A | 19 | 0.0036 | 0.62 | 0.28 | .03 | |
| ACOT11 /LOC105378734 | rs12752888_C | 1 | 0.0035 | ‐0.47 | 0.18 | <.01 | |
| MTRR | rs1801394_G | 5 | 0.0029 | 0.80 | 0.23 | <.01 | |
| DAB1 | rs1539053_A | 1 | 0.0017 | 0.50 | 0.20 | .01 | |
| MMP3 | rs948399_C | 11 | 0.0011 | 0.41 | 0.17 | .01 | |
| Model II | |||||||
| APOE | rs429358_C | 19 | 0.0042 | ‐0.59 | 0.23 | .01 | |
| ACOT11 / LOC105378734 | rs12752888_C | 1 | 0.0024 | ‐0.37 | 0.15 | .01 | |
| TOMM40 | rs8106922_G | 19 | 0.0011 | ‐0.31 | 0.16 | .05 | |
| SLCO1B1 | rs4363657_C | 12 | 0.0008 | 0.38 | 0.16 | .02 | |
| MMP3 | rs948399_C | 11 | 0.0003 | 0.29 | 0.15 | .05 |
Notes: Statistically significant genes are shown in association with cognitive frailty compared to healthy adults Models I and II use Mini‐Mental State Examination (MMSE) and Trail Making Tests (TMT) parameters, respectively, to define cognitive frailty. Bold text indicates the closest gene to an intergenic single nucleotide polymorphism (SNP). The xgboost rank importance: xgboost ranks each SNP by level of importance based on its contribution to the model. Beta coefficients, standard error (SE), and P‐values for each SNP were derived from subsequent logistic regression analysis after xgboost ranking.
3.2. Medication genetic variants and risk
One of the interesting genomic findings was the SLCO1B1 rs4363657 allele C (P = .02) in predictive Model II. SLCO1B1 has been associated with the metabolite X12063, both of which are markers of lean muscle mass loss. 27 SLOCO1B1 has been linked to drug metabolism that results in higher blood concentrations of statins. 28 SLOCO1B1 is essential for drug hepatic uptake and the C variant is associated with reduced OATP1B1 activity. OATP1B1 can facilitate drug uptake at the blood‐brain barrier and may lead to drug toxicity in the central nervous system. 29 Additionally, anticholinergic medications were significantly associated with both attention (TMT‐A), executive functioning (TMT‐B), and global cognition for individuals with cognitive frailty. Anticholinergic drug burden (ACB) was ranked as one of the top predictors of cognitive frailty in both models. A detailed description and analysis of the relationship between ACB and cognitive frailty is available in a separate publication. 11
3.3. Neuroinflammatory cytokine markers
This study found elevated levels of neuroinflammatory cytokines, specifically interleukins IL1, IL6, IL6sR 1&2, TNF‐alpha, ESR, and TNFsR1&2 in association with cognitive frailty. Additionally, participants with cognitive frailty had higher levels of resistin (P < .0001) compared to healthy adults in both models; notably, resistin regulates IL‐6, TNF, and C‐reactive protein (hs‐CRP). 30 Both fibrinogen (P < .0001) and advanced glycation end product (AGE; P < .0001) were found to be elevated. Such increases in AGE have been linked to oxidative stress and high levels of alpha‐2 globulin (A2M; P < .0001) and alpha‐1 globulin (A1M; P < .0001). A2M and A1M are protease inhibitor cytokine transporters, whose aberrant expression has been linked to AD, and in this study, were also found in participants with cognitive frailty, but not in healthy older adults. 31 TNF‐related apoptosis‐inducing ligand (TRAIL) was found to be lower (P < .0001) in individuals with cognitive frailty. Lower serum TRAIL levels are associated with a decrease in cellular apoptosis and an increased risk of stroke and cardiovascular disease. 32 Taken together, these findings support the theory of chronic neuroinflammation as playing a role in neuro‐immuno‐endocrine dysfunction that may contribute to cognitive frailty. 33
3.4. Nutrient and lipid metabolism
We found distinct nutrient and lipid biomarkers were predictive of cognitive frailty. Specifically, the following nutrient pattern associated with cognitive frailty included lower levels of vitamin E alpha tocopherol (P < .0001), albumin (P < .0001), omega‐6 and 3 (P < .0001) were found in cognitively frail older adults with global cognitive decline and lower vitamin B6 (P < .0001); albumin (P < .0001), omega‐6 and 3 (P < .001) with poor attention and executive functioning. Low vitamin E alpha tocopherol was associated with global cognitive decline (P < .0001) and poor attention (P = .037) but not executive functioning. Interestingly, a second association pattern was characterized by low trans fats measured by low‐ and high‐density lipoprotein (LDL and HDL). Frail older adults with poor global cognitive decline had lower levels of LDL (P < .0001) and HDL (P < .047) than healthy older adults. Overall, significantly lower levels of these lipids were present for older adults with cognitive frailty compared to healthy older adults.
3.5. Metabolites
Metabolomic ceramides C16:0, C20:0, C20:5, C24:0, and C:22:0 markers were predictive of cognitive frailty in both analytic models; with significant differences in C20:0 (P = .041), C20:5 (P < .0001) and C16:0 fatty acid weight (P = .008) fatty acid area ( P = .013) between cognitively frail and healthy older adults. Ceramides C16:0 and C20:0 have been associated with greater risk of impairment in attention as measured with the TMT‐A, 34 and in this study, we found C20:5 to be lower in older adults with cognitive frailty and predictive for attention deficits as measured by TMT‐A (P = .028) but not executive function. Additionally, serum ceramides varied with some high and others low supporting the finding that timing and onset of memory impairment may be a factor affecting levels. 34
3.6. Renal function
Poor renal function was predictive of cognitive frailty; with lower 24‐hour urinary creatinine (P < .0001), higher blood urea nitrogen (BUN; P < .0001), higher urine proteins (P = .03) and nitrates (P < .0002), higher serum creatinine (P = .022), lower serum and urinary calcium (P < .001), higher uric acid (P < .01), and higher cystatin C (P < .0001) than healthy older adults. Taken together, markers of poor renal function have been linked to changes in mobility disability 35 with higher cystatin C associated with increased likelihood of converting from MCI to AD. 36
3.7. Hematologic/immune function
Iron deficient anemia was associated with individuals with cognitive frailty as noted by low hemoglobin (P < .0001), high red cell distribution width (RDW; P < .0001), low mean corpuscular hemoglobin (MCH) concentration (P < .0001), high soluble transferrin receptor (P = 0.01), and low mean platelet volume (MPV; P = .08). Furthermore, immune dysfunction was indicated by high white blood cell count (WBC; P = .007), low lymphocytes (P < .0001), high neutrophils (P < .0001), and low CD14 (P < .0001). Collectively, these findings support a theory of immune system dysfunction seen in both predictive models suggesting individuals with cognitive frailty have a decreased humoral immune response. 37
3.8. Endocrine/hormone function
Dehydroepiandrosterone sulfate (DHEA), testosterone, and urinary cortisol were found to be low for those with cognitive frailty compared to healthy adults (P < .001). DHEA has been found to inhibit IL‐6, thus providing a connection between endocrine and immune function. 38 , 39 , 40 Another interesting finding was the connection between biomarkers of nutrition, low fatty acid levels, and high levels of c‐terminal telopeptide of type‐1 collagen l (PINP; P < .0001) and parathyroid hormone (PTH; P < .0001) in association with cognitive frailty. Both PINP and PTH have been linked to low levels of vitamin D (P < .0001), which we also found in our participants with cognitive frailty. 41 Total insulin‐like growth factor, plasma insulin (P = .04), and free thyroxine fT4 were low in individuals with cognitive frailty (P < .0001). Methylmalonic acid (MMA) is linked to high levels of vitamin B12 and high levels of homocysteine (P < .0001) together with the MTRR SNP rs1801394, all of which share the same pathway and are predictive of cognitive frailty. This pathway interaction has been linked to both cognitive performance and increased risk for physical frailty. 26 , 42
Figures 2 and 3 summarize the top SNPs and protein biomarkers ranked by the level of importance in predicting cognitive frailty. These feature importance scores were generated during statistical analysis in xgboost.
4. DISCUSSION
When the results are interpreted by individual biological pathways, several proposed theories on AD are supported including (1) immunological system dysfunction, (2) environmental exposures and toxicities (ie, ACB), (3) genetic factors, and (4) chronic neuroinflammation. 37 In contrast, when the results are summarized using the updated hypothesis of multi‐system dysfunction, the findings closely align with the aging theory of cellular senescence. 12 , 43 , 44 Cellular senescence theory is based on the concept of biological cellular damage and aberrant gene expression leading to loss of cell function and age‐related disease. 7 , 9 , 43 For example, participants with cognitive frailty had higher levels of inflammatory protein markers including; hs‐CRP, resistin, and A2M compared to healthy older adults. Resistin regulates IL‐6, TNF, IL‐1, and A2M which are protease inhibitor cytokine transporters linked to AD. 30 , 31 These findings highlight specific pathway interactions for neuroinflammatory cytokines coupled with gene SNPs associated with cellular senescence; eg, MMP3 rs948399 and CD33 rs3865444. Functional studies in AD have shown when CD33 is overexpressed, microglia‐mediated neuroinflammation pathways are activated and amyloid beta (Aβ) phagocytosis is inhibited. 45 , 46 Additional markers of cellular senescence in this study include metabolomic ceramides C16:0, C20:0, and C20:5. Ceramides regulate cellular proliferation and apoptosis and have been positively correlated with the number of Aβ plaques on post mortem biopsy. 34 At low levels, ceramides regulate cellular proliferation and apoptosis; at high levels they inhibit cell division and are intermediates of inflammatory cytokines and subclinical atherosclerosis. 47 Together, these biological markers define the senescence‐associated secretor phenotype (SASP). 44 While cellular senescence in peripheral tissues has been linked to aging and age‐related diseases, its involvement in neurogenerative diseases and AD is still being explored. 44 , 48
The findings from the proposed models herein allows hypothesis testing for additional study including (1) examining specific pathway associations with significant SNPs and protein biomarkers to determine whether the identified genes are clinically relevant; (2) exploring gene variant risk of medication metabolism, thereby expanding upon findings in this study to better understand the relationship between a group of high‐risk medications (ACB) and specific gene variants associated with drug metabolism; 11 , 49 and (3) understanding the role of cellular senescence in cognitive frailty.
Nevertheless, challenges still exist in generating hypothesis testing based on our models. Generalizability may be limited due to the cross‐sectional analysis of existing data with a primarily homogeneous European population. Additionally, the systems model proposed in this study accounts for known human genetic, clinical, and laboratory data; yet, systems models must also be able to suggest causal roles for the findings and ultimately offer interventions. 48 , 50 At this time, our results should be replicated in other large studies with similar biomarker measures such as the Alzheimer's Disease Neuroimaging Initiative (ADNI). Although ML methods are promising for reducing complex biological systems and characterizing key contributing variables, the results presented in this study do not provide insight into the dynamic relationship of multisystem dysfunction and AD over time. Future systems models should include longitudinal observational studies to better understand patterns of biomarker change associated with AD, elucidate disease progression, and examine the direction and rate of change over time. One way to accomplish this goal is to use ML statistical analysis to model changes in protein, genetic, and neuroimaging markers in existing population‐based studies. Innovative data sharing and collaborations such as the Integrative Analysis of Longitudinal Studies on Aging (IALSA) will be important for generalizability and modeling of complex biological interactions in future dementia studies. Considering the progressive course of AD, studies that use ML predictive analytic methods to model biomarker changes longitudinally will be essential. This effort will require specialized training for research teams, collaborative efforts between computer science and basic science disciplines, and epidemiological studies with consistent biomarker measures over the participants’ lifetime. Our results support the theoretical framework of cognitive frailty as a complex system when modeled with a ML statistical approach (Figure 1). Fortunately, analytical tools are now available to explore complex aging syndromes that may be pivotal in shedding light on the multiple dimensions of cognitive frailty.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Supplementary appendix contains methodological and analytical details for the study results discussed in this manuscript. All documents on the population predictive model results, statistical methods, and reproducible R code are available at GitHub Laboratory of Neurogenetics National Institute of Aging NIH, Population Predictive Model InCHIANTI Study repository; https://github.com/neurogenetics/Population-Predictive-Model-InCHIANTI-Study.
ACKNOWLEDGMENTS
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging. We thank Christopher Bickel, BA for the artistic interpretation provided for the theoretical framework of cognitive frailty as a complex system.
Sargent L, Nalls M, Amella EJ, et al. Shared mechanisms for cognitive impairment and physical frailty: A model for complex systems. Alzheimer's Dement. 2020;6:e12027 10.1002/trc2.12027
Funding information
The authors received no specific funding for this work.
REFERENCES
- 1. Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726‐734. [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146‐56. [DOI] [PubMed] [Google Scholar]
- 3. Paganini‐Hill A, Clark LJ, Henderson VW, Birge SJ. Clock drawing: analysis in a retirement community. J Am Geriatr Soc. 2001;49(7):941‐947. [DOI] [PubMed] [Google Scholar]
- 4. Han ES, Lee Y, Kim J. Association of cognitive impairment with frailty in community‐dwelling older adults. Int Psychogeriatr. 2014;26(1):155‐163. [DOI] [PubMed] [Google Scholar]
- 5. Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80(22):2055‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health‐and cognitive‐related outcomes in older age: from epidemiology to prevention. J Alzheimer's Dis. 2018;62(3):993‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Searle SD, Rockwood K. Frailty and the risk of cognitive impairment. Alzheimers Res Ther. 2015;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69(12):1536‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song X, Mitnitski A, Rockwood K. Age‐related deficit accumulation and the risk of late‐life dementia. Alzheimers Res Ther. 2014;6(5‐8):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rockwood K. Lessons from mixed dementia. Int Psychogeriatrics. 1997;9(3):245‐249. [DOI] [PubMed] [Google Scholar]
- 11. Sargent L, Nalls M, Amella EJ, Mueller M, Lageman SK, Bandinelli S, Colpo M, Slattum PW, Singleton A, & Ferrucci L. Anticholinergic drug induced cognitive and physical impairment: Results from the InCHIANTI study. J Gerontol: Series A. 2020;75(5):995–1002. 10.1093/gerona/gly289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14(6):709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odden MC, Melzer D. Machine learning in aging research. J Gerontol A Biol Sci Med Sci. 2019;74(12):1901‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sargent L, Nalls M, Starkweather A, et al. Shared biological pathways for frailty and cognitive impairment: a systematic review. Ageing Res Rev. 2018;47:149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mekli K, Nazroo JY, Marshall AD, Kumari M, Pendleton N. Proinflammatory genotype is associated with the frailty phenotype in the English Longitudinal Study of Ageing. Aging Clin Exp Res. 2016;28(3):413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Bryant SE, Xiao G, Barber R, et al. A serum protein‐based algorithm for the detection of Alzheimer's disease. Arch Neurol. 2010;67(9):1077‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solfrizzi V, Scafato E, Lozupone M, et al. Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: the Italian Longitudinal Study on Aging. Am J Geriatr Psychiatry. 2017;25(11):1236‐1248. [DOI] [PubMed] [Google Scholar]
- 18. Ashendorf L, Jefferson AL, Connor MKO, et al. Trail making test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol. 2008;23(2):129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychologial Tests: Adiminstration, Norms, and Commentary. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 20. Delrieu J, Andrieu S, Pahor M, et al. Neuropsychological profile of “Cognitive Frailty” subjects in MAPT Study. J Prev Alzheimers Dis. 2016;3(3):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage, Oxford, England, UK: Southern Universities Press; 1958. [Google Scholar]
- 22. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618‐1625. [DOI] [PubMed] [Google Scholar]
- 23. Stenholm S, Ferrucci L, Vahtera J, et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies. J Gerontol A Biol Sci Med Sci. 2019;74(5):667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen T, Guestrin C. XGBoost: A scalable tree boosting system In: Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. Vol 13‐17‐Augu. New York, NY, USA: Association for Computing Machinery; 2016:785‐794. 10.1145/2939672.2939785. [DOI] [Google Scholar]
- 25. Purcell S, Neale B, Todd‐Brown K, et al. PLINK: a toolset for whole‐genome association and population‐based linkage analysis. Am J Hum Genet. 2007;81(3):559‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matteini AM, Walston JD, Fallin MD, et al. Markers of B‐Vitamin deficiency and frailty in older women. J Nutr Heal Aging. 2008;12(5):303‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korostishevsky M, Steves CJ, Malkin I, Spector T, Williams FM, Livshits G. Genomics and metabolomics of muscular mass in a community‐based sample of UK females. Eur J Hum Genet. 2016;24(2):277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed S, Zhou Z, Zhou J, Chen SQ. Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genomics Proteomics Bioinforma. 2016;14(5):298‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8(7):787‐802. [DOI] [PubMed] [Google Scholar]
- 30. Kizilarslanoglu MC, Kara O, Yesil Y, et al. Alzheimer's disease, inflammation, and novel inflammatory marker: resistin. Turkish J Med Sci. 2015;45(5):1040‐1046. [PubMed] [Google Scholar]
- 31. Ashton NJ, Kiddle SJ, Graf J, et al. Blood protein predictors of brain amyloid for enrichment in clinical trials. Alzheimers Dement (Amst). 2015;1(1):48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang YH, Park MG, Noh KH, et al. Low serum TNF‐related apoptosis‐inducing ligand (TRAIL) levels areassociated with acute ischemic stroke severity. Atherosclerosis. 2015;240(1):228‐233. [DOI] [PubMed] [Google Scholar]
- 33. Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head‐to‐toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041‐2056. [DOI] [PubMed] [Google Scholar]
- 34. Mielke MM, Bandaru VVR, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu CK, Lyass A, Massaro JM, D'Agostino RB, Fox CS, Murabito JM. Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: the Framingham Offspring Study. J Gerontol A Biol Sci Med Sci. 2014;69 A(3):301‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopez OL, Kuller LH, Mehta PD, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70(19 part 1):1664‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trevisan K, Cristina‐Pereira R, Silva‐Amaral D, Aversi‐Ferreira TA. Theories of aging and the prevalence of Alzheimer's disease. Biomed Res Int. 2019;2019:9171424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6‐minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56(3):454‐461. [DOI] [PubMed] [Google Scholar]
- 39. Kipper‐Galperin M, Galilly R, Danenberg HD, Brenner T. Dehydroepiandrosterone selectively inhibits production of tumor necrosis factor alpha and interleukin‐6 [correction of interlukin‐6] in astrocytes. Int J Dev Neurosci. 1999;17(8):765‐775. [DOI] [PubMed] [Google Scholar]
- 40. Arlt W, Hewison M. Hormones and immune function: implications of aging. Aging Cell. 2004;3(4):209‐216. [DOI] [PubMed] [Google Scholar]
- 41. Alvarez‐Ríos AI, Guerrero JM, García‐García FJ, et al. Associations between frailty and serum N‐terminal propeptide of type I procollagen and 25‐hydroxyvitamin D in older Spanish women: the Toledo Study for Healthy Aging. Exp Gerontol. 2015;69:79‐84. [DOI] [PubMed] [Google Scholar]
- 42. Nurk E, Refsum H, Bjelland I, et al. Plasma free choline, betaine and cognitive performance: the Hordaland Health Study. Br J Nutr. 2013;109(3):511‐519. [DOI] [PubMed] [Google Scholar]
- 43. Kritsilis M, V Rizou S, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. 2018;19(10):pii: E2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hooli B, Tanzi RE. The genetic basis of Alzheimer's disease: findings from genome‐Wide Studies Genomics, Circuits, and Pathways in Clinical Neuropsychiatry, New York, NY: Elsevier Inc:547‐571. [Google Scholar]
- 46. Suwannasom N, Smuda K, Kloypan C, et al. Detection of CD33 expression on monocyte surface is influenceby phagocytosis and temperature. Gen Physiol Biophys. 2019;38(5):369‐378. [DOI] [PubMed] [Google Scholar]
- 47. Summers S. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42‐72. [DOI] [PubMed] [Google Scholar]
- 48. Fossel M. A unified model of dementias and age‐related neurodegeneration. Alzheimer's Dement. 2020;16(2):365‐383. [DOI] [PubMed] [Google Scholar]
- 49. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia. JAMA Intern Med. 2015;175(3):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khachaturian ZS, Mesulam M‐M, Khachaturian AS, Mohs RC. The special topics section of Alzheimer's and dementia. Alzheimer's Dement. 2015;11(11):1261‐1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix contains methodological and analytical details for the study results discussed in this manuscript. All documents on the population predictive model results, statistical methods, and reproducible R code are available at GitHub Laboratory of Neurogenetics National Institute of Aging NIH, Population Predictive Model InCHIANTI Study repository; https://github.com/neurogenetics/Population-Predictive-Model-InCHIANTI-Study.


