Glioblastoma (GBM) are aggressive brain tumors that cannot be cured due to genetic and epigenetic heterogeneity, and the presence of glioma stem cells [1, 3–5, 7, 8]. Neftel and colleagues recently reported that malignant cells in GBM exist in four main cellular states recapitulating distinct neural cell types, are influenced by the tumor microenvironment, and exhibit state-transition or plasticity [6]. Because spatial information is lost during the preparation of single cells for sequencing, we sought to determine if cellular states may correspond to topographical location within the tumor. We extracted gene expression profiles of cellular states and proliferation from the study, and applied this data to the five anatomical structures in the IvyGAP GBM database (https://glioblastoma.alleninstitute.org/). Strikingly, we found that these newly defined cellular states are indeed enriched in specific anatomical locations within GBM.
Five anatomical regions were analyzed within GBM by the IvyGAP project (Figure 1A): Leading Edge (LE), the outermost boundary of the tumor with extremely rare glioma cells; Infiltrating Tumor (IT), an intermediate zone between LE and the core containing 10–20% neoplastic cells; Cellular Tumor (CT) core with few non-neoplastic cells; Pseudopallisading cells Around Necrosis (PAN) where glioma cells aggregate around necrotic zones; and Microvascular Proliferation (MVP). To test the hypothesis that cellular states correspond to a spatial “address”, we compared expression of the “cellular state” genes identified by Neftel and colleagues in the five anatomical locations using ANOVA analysis. Importantly, a group of eight commonly used “housekeeping” genes (ACTB, B2M, GAPDH, GUSB, PPIA, RPLP0, TBP) did not show significant association with any feature. In addition, expression of cellular state signatures in MVP, a non-malignant feature of the tumor comprised of blood vessels, were strongly positive for the G1/S and G2/M proliferation signatures but either neutral or negatively correlated with the four malignant features (Figure 1B). In contrast, malignant cellular state signature genes were all predictive of one or more anatomical regions.
Figure 1.
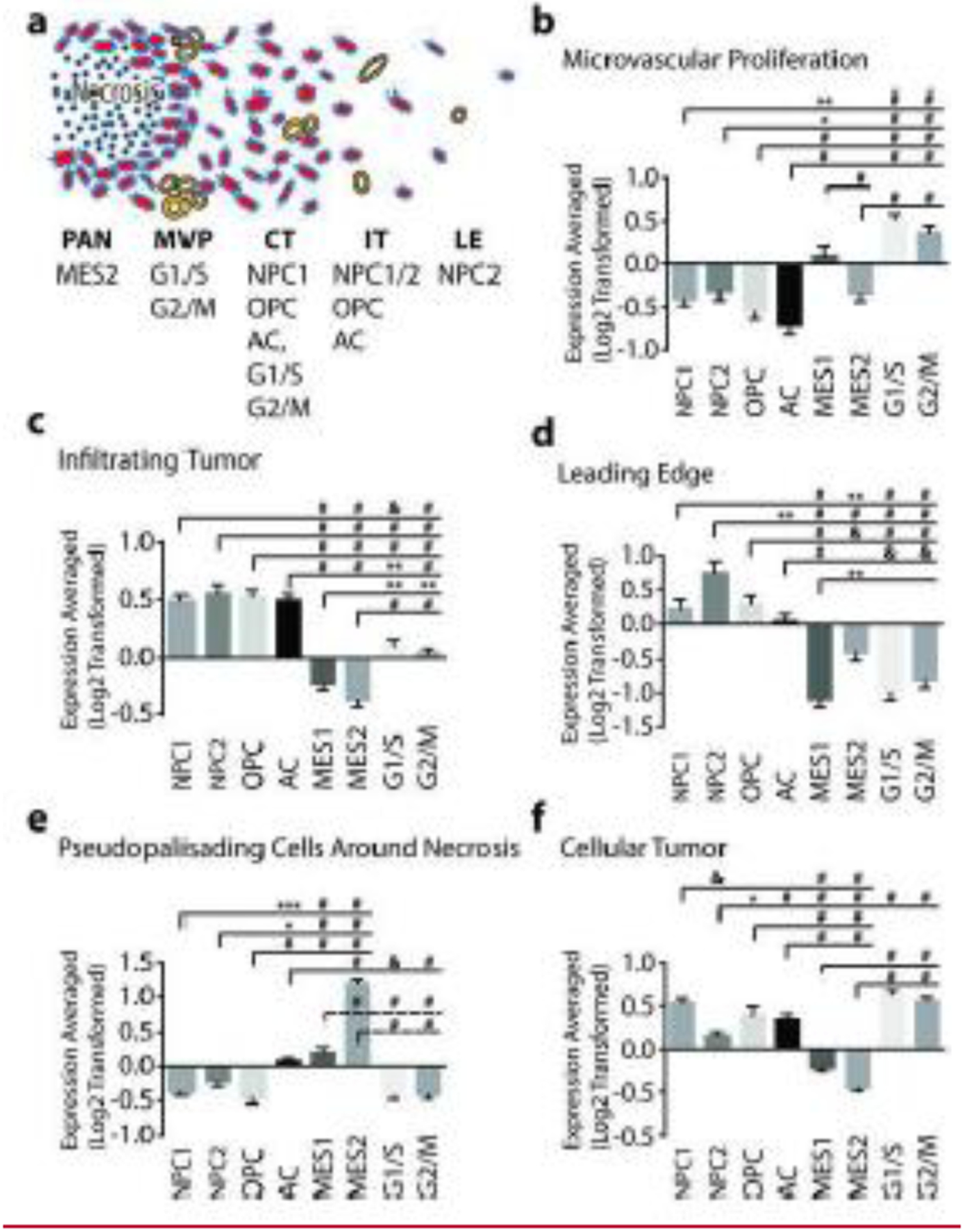
Cellular States are Enriched in Specific Anatomical Features of Glioblastoma. (a) Summary of the enriched cellular states in the five anatomical regions analyzed within GBM by the IvyGAP project. Averaged, log2-transformed, expression of cellular state signature genes in (b) - Microvascular proliferation (MVP). (c) Infiltrating Tumor (IT); (d) - Leading Edge (LE); (e) - Pseudopallisading cells around necrosis (PAN); and (F) - Cellular Tumor (CT). Abbreviations: NPC - Neural Precursor-Like, OPC - Oligodendrocyte Precursor-Like, AC - Astrocyte-Like, MES - Mesenchymal-Like. Statistics: One-way ANOVA with Tukey’s multiple comparisons test. * p<0.05, ** p<0.01, & p<0.001, # p<0.0001.
Together, the LE and IT constitute the main invasive interface or boundary between tumor and normal brain. IT is enriched in signatures of neural (NPC) and oligodendroglial progenitors (OPC), as well as astrocytic differentiation (AC). In contrast, proliferation signatures are either neutral or very weak in this feature, suggesting these tumor regions contain fewer cycling cells (Figure 1C, D). Similarly, the leading-edge feature is enriched in the NPC and OPC signatures but unlike IT lacked the AC signature (Figure 1D). In addition, the MES1 signature was strongly inhibited in the LE as compared to all other regions. Finally, given the reduced cellularity in IT and LE regions, we cannot rule out the potential contribution of non-neoplastic microenvironmental elements to the above signature correlations.
PAN is generally found in the core of tumors, and we have previously suggested it represents a hypoxic glioma stem-cell niche [2]. PAN is the main anatomical feature containing the hypoxia dependent MES2 signature (Figure 1E), while negative G1/S and G2/M signatures suggest slower proliferation around necrosis. In contrast, CT in the GBM core is strongly enriched in proliferative signatures and represents the rapidly dividing powerhouse of the glioma. In addition, this feature shows strong negative MES1 and MES2 signatures, highlighting the specificity of the MES gene signatures for hypoxic regions of the tumor. In contrast, the CT showed a 3.4 fold higher NPC1 over NPC2 score. NPC1 and NPC2 signatures reflect tendencies to differentiate into OPCs or neurons, respectively [6], and it appears that CT shows greater oligodendroglial differentiation by NPCs, while in LE neuronal differentiation is favored.
We used ANOVA to determine which of the signature genes best predicts both cellular state and anatomical location. We arbitrarily set a threshold of eight out of ten possible comparisons between the five anatomical features to be significant to identify genes that are highly predictive. This analysis identified TAGLN3, APOD GFAP, and ANXA1 to be best predictors of the NPC, OPC, AC, and MES states, respectively. Testing these genes as potential reporters of specific cellular state and address may prove to be a powerful new tool for studying cellular plasticity in preclinical studies.
Funding:
NIH R01 CA187780, NIH R21 NS106553
None of the authors has a material conflict of interest to disclose.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bar EE (2011) Glioblastoma, cancer stem cells and hypoxia. Brain Pathol 21: 119–129 Doi 10.1111/j.1750-3639.2010.00460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG (2010) Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol 177: 1491–1502 Doi S0002-9440(10)60201-510.2353/ajpath.2010.091021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69–82 Doi S1535-6108(06)00369-210.1016/j.ccr.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 4.Dirks PB (2010) Brain tumor stem cells: the cancer stem cell hypothesis writ large. Mol Oncol 4: 420–430 Doi 10.1016/j.molonc.2010.08.001S1574-7891(10)00082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE et al (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501–513 Doi S1535-6108(09)00087-710.1016/j.ccr.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM et al (2019) An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178: 835–849 e821 Doi 10.1016/j.cell.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111 [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63: 5821–5828 [PubMed] [Google Scholar]


