Dear Editor,
In the course of Coronavirus Disease 2019 (COVID-19)-related acute hypoxemic respiratory failure (AHRF), nasal high flow (NHF) has been initially seldom used [1]. Reassessment of environmental contamination risk progressively led to broader NHF application [2, 3]. Our purpose was to evaluate the ROX index [4], defined as the ratio of SpO2/FiO2 to respiratory rate (RR), as an early marker of NHF response and a potential predictor of its failure in the ICU setting.
In this single-center retrospective study, all 18-year-old or older patients admitted to the ICU during the peak of the COVID-19 outbreak were screened for eligibility. Participants presenting with AHRF related to SARS-CoV-2 infection (confirmed by molecular testing) and treated with NHF as first-line ventilatory support were included. Patients’ characteristics and NHF-related data were collected from admission until NHF weaning or intubation which defined NHF failure. The ROX index was recorded several times daily. Local Ethics Committee approved the study. Participants were informed of the research’s purpose and their right to decline participation. Statistical analysis included association between early response to NHF [i.e., the latest value of the ROX index within the first 4 h after NHF initiation (ROX-H0H4)] and risk for intubation (Cox’s model for patients still at risk at H4). Maximization of the Youden’s index led to an optimal cut-off of the ROX index to predict NHF outcome.
Among all 116 consecutive patients admitted to ICU from March 8 to April 16, 2020, 32 were not COVID-19-related, 20 were intubated prior to admission and 2 declined participation. Median age of the study population (N = 62) was 55 (IQR 48–63). Patients presented with profound hypoxemia at NHF initiation [median FiO2 and SpO2 were 0.8 (IQR 0.6–1) and 96% (IQR 94–98), respectively] with median RR of 25 breaths per minute (IQR 21–32). Initial NHF settings were: FiO2: 0.8 (0.6–1) and gas flow: 50 L/min (40–60). Twenty-one patients (34%) succeeded on NHF and were discharged from ICU, whereas 39 (63%) required MV and 2 (3%) died while under NHF because of do-not-intubate order (they were excluded from further analysis). Overall ICU mortality was 17%.
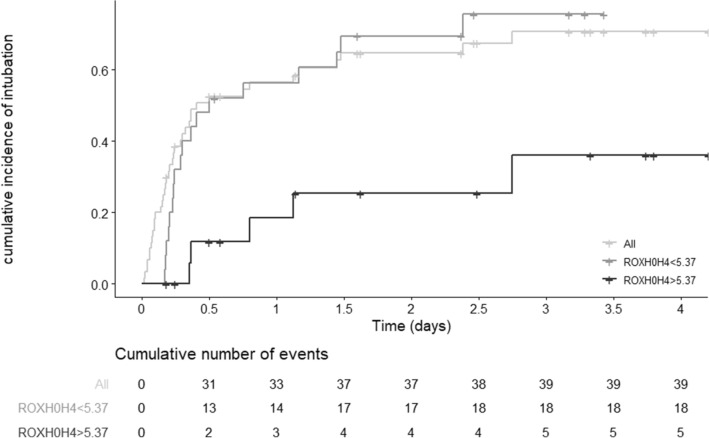
Median time to intubation was 10 h (95% CI 7–57). Kaplan–Meier estimates of risk for intubation (N = 60) is illustrated in Fig. 1. Median ROX-H0H4 was 5.4 (IQR 3.9–7.1). In Cox’s model, ROX-H0H4 ≥ 5.37 was significantly associated with a lower risk for intubation after H4 (HR 0.59, 95% CI 0.41–0.84; P = 0.0037) for patients still at risk (N = 45). ROX-H0H4 demonstrated a good discrimination (area under the ROC curve 0.75, 95% CI 0.6–0.9; sensitivity 0.66, specificity 0.83).
Fig. 1.
Cumulative incidence of orotracheal intubation for all at-risk patients (N = 60) and after 4 h of NHF (N = 45) stratified on ROX-H0H4 (landmark at H4). NHF, nasal high flow. ROX-H0H4 was defined as the latest value of the ROX index within the first 4 h after NHF initiation
In conclusion, early application of NHF as first-line ventilatory support during COVID-19-related AHRF may have obviated the need for intubation in up to a third of cases. In this circumstance, the ROX index measured within the first 4 h after NHF initiation could be an easy-to-use marker of early ventilatory response. Its most accurate cut-off was slightly higher than previously validated in AHRF [4], probably because of specific ventilatory adaptation observed in COVID-19-related AHRF [5]. Despite limitations inherent to the study’s retrospective design, our results suggest that the ROX index could help identify patients who will fail on NHF, in order not to further delay intubation.
Acknowledgements
Contributors: Dan Longrois, MD, PhD, Assistance Publique-Hôpitaux de Paris, Department of Anesthesiology, Hôpital Louis Mourier, F-92700, Colombes, France. Didier Dreyfuss, MD, Sorbonne Université, INSERM Unit S_1155 (CoRaKid), Paris, France.
Authors contributions
JDR, NZ, JM designed the study. NZ, DR, DL acquired the data. JDR, NZ, JM, OR analyzed and interpreted the data. NZ, JDR drafted the manuscript. JM, OR, DR, DD, DL provided significant input in the writing of the manuscript.
Funding
No funding was used specifically for this study.
Compliance with ethical standards
Conflicts of interest
JDR received travel expenses and accommodation coverage from Fisher & Paykel Healthcare to attend scientific meetings. Fisher & Paykel Healthcare provided support for the ongoing High Flow ACRF trial (NCT03406572) but took no part in design or conduct of the study. OR received speaker fees from Air Liquide. His institution received consultancy fees from Hamilton Medical. DR received personal fees from Astellas. Other authors have no conflict of interest.
Ethical approval
Local Ethics Committee (CEERB Paris Nord (IRB 00006477) approved the study. Participants were informed of the purpose of the research and of their right to decline participation. The study was registered at Clinicaltrials.gov (NCT04385823).
Footnotes
The Contributors are listed in the acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jean-Damien Ricard, Email: jean-damien.ricard@aphp.fr.
Contributors:
References
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 5.Ottestad W, Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]