Abstract
Fanconi anemia (FA) is a rare genetic disorder characterized by bone marrow failure, predisposition to cancer, and congenital abnormalities. FA is caused by pathogenic variants in any of 22 genes involved in the DNA repair pathway responsible for removing interstrand crosslinks. FANCL, an E3 ubiquitin ligase, is an integral component of the pathway, but patients affected by disease-causing FANCL variants are rare, with only nine cases reported worldwide. We report here a FANCL founder variant, anticipated to be synonymous, c.1092G>A;p.K364=, but demonstrated to induce aberrant splicing, c.1021_1092del;p.W341_K364del, that accounts for the onset of FA in thirteen cases from South Asia, twelve from India and one from Pakistan. We comprehensively illustrate the pathogenic nature of the variant, provide evidence for a founder effect, and propose including this variant in genetic screening of suspected FA patients in India and Pakistan, as well as those with ancestry from these regions of South Asia.
Keywords: FANCL, founder variant, Fanconi anemia, India, South Asia
Fanconi anemia (FA) is a rare, mostly recessive, genetic disorder caused by a DNA repair deficiency which fails to coordinate the removal of interstrand crosslinks, leading to increased genomic instability. FA is characterized by the development of progressive bone marrow failure resulting in aplastic anemia, congenital skeletal malformations, developmental delays, and an increased predisposition to solid tumors and hematological malignancies (Faivre et al., 2000; Kottemann & Smogorzewska, 2013). The onset of hematologic disease occurs at an average age of 7 years (Butturini et al., 1994; Faivre et al., 2000; Kutler et al., 2003), with varying hematologic severity between FA-causing genes and even among specific variants within the same gene (Gillio, Verlander, Batish, Giampietro, & Auerbach, 1997). FA is caused by a wide spectrum of distinct pathogenic variants across 22 FANC genes (Knies et al., 2017; Mamrak, Shimamura, & Howlett, 2017), and although the frequency of FA-causing genes may vary by region or population, as noted in a recent study identifying FANCC patients as rather rare in Japan (Mori et al., 2019), FANCA (64%), FANCC (12%), and FANCG (8%) are the three most commonly observed disease-causing genes among FA patients, accounting for 84% of cases worldwide (Wang & Smogorzewska, 2015).
FA is a genetically heterogeneous disorder (Kimble et al., 2018), but even amidst the genetic diversity observed among FA patients, high frequency pathogenic variants in FA genes influenced by a founder effect have been identified among genetically isolated populations. But, to date, these variants were limited to the more common FA genes. Examples include FANCA variants among Spanish Gypsies (c.295C>T;p.Q99*) (Castella et al., 2011) and Japanese (c.2546delC) (Mori et al., 2019); FANCC variants among Ashkenazi Jews (c.456+4A>T) (Verlander et al., 1995) and Dutch/Manitoba Mennonites (c.67delG) (de Vries et al., 2012); and FANCG variants among the Portuguese-Brazilian (c.1077–2A>G), French-Acadian (c.1480+1G>C), and Korean/Japanese (c.307+1G>C) populations (Auerbach et al., 2003).
FANCL (MIM# 608111) is an E3 ubiquitin ligase and is a component of the multiprotein core complex in the FA DNA repair pathway (Meetei et al., 2003; Walden & Deans, 2014). FANCL interacts with UBE2T (FANCT), which serves as the E2-ubiquitin conjugating enzyme, and together they participate in the transfer of ubiquitin moieties to FANCD2 and FANCI. Ubiquitylation of FANCD2 and FANCI is necessary to activate the subsequent steps of the FA pathway which repair the damage caused by interstrand crosslinks in DNA (Walden & Deans, 2014). Worldwide, the total percentage of FA cases caused by pathogenic FANCL variants amounts to only 0.2%−0.4% (Neveling, Endt, Hoehn, & Schindler, 2009; Wang & Smogorzewska, 2015), with only 9 reported cases (Ali et al., 2009; Ameziane et al., 2012; Chandrasekharappa et al., 2013; Meetei et al., 2003; Vetro et al., 2015; Wu et al., 2017), making it a very rare FA group.
We present here thirteen patients with a pathogenic FANCL variant, NC_000002.11:g.58387243C>T (hg19), initially predicted to be synonymous, NG_007418.1(NM_018062.3):c.1092G>A; p.K364=, but shown to induce aberrant mRNA splicing, c.1021_1092del. Twelve patients are homozygous and one is a compound heterozygote; twelve are from India and one is from Pakistan. This variant has only been found in individuals of South Asian ancestry and reveals a founder effect in our patients.
Patients IN01-IN10 were enrolled at the Department of Cytogenetics of the National Institute of Immunohaematology (NIIH) in Mumbai, India. Patient TKFA33, Pakistani in origin, was diagnosed at Tokai University Hospital in Kanagawa, Japan. FA17, whose pathogenic variant was previously reported (Chandrasekharappa et al., 2013), and FA91, both of Indian origin, were enrolled in the International Fanconi Anemia Registry (IFAR) at the Rockefeller University in New York, USA. The study was carried out with consent of FA patients or parents, as appropriate. The study protocols were approved by the Institutional Ethics Committee (IEC) for human subjects in NIIH (Mumbai), Tokai University, or Kyoto University. The Institutional Review Board of the Rockefeller University, New York, USA, had approved the studies involving FA17 and FA91, while The Office of Human Subjects Research at the National Institutes of Health and Institutional Review Board of the National Human Genome Research Institute (NHGRI) approved the analysis of de-identified DNA samples from The Rockefeller University.
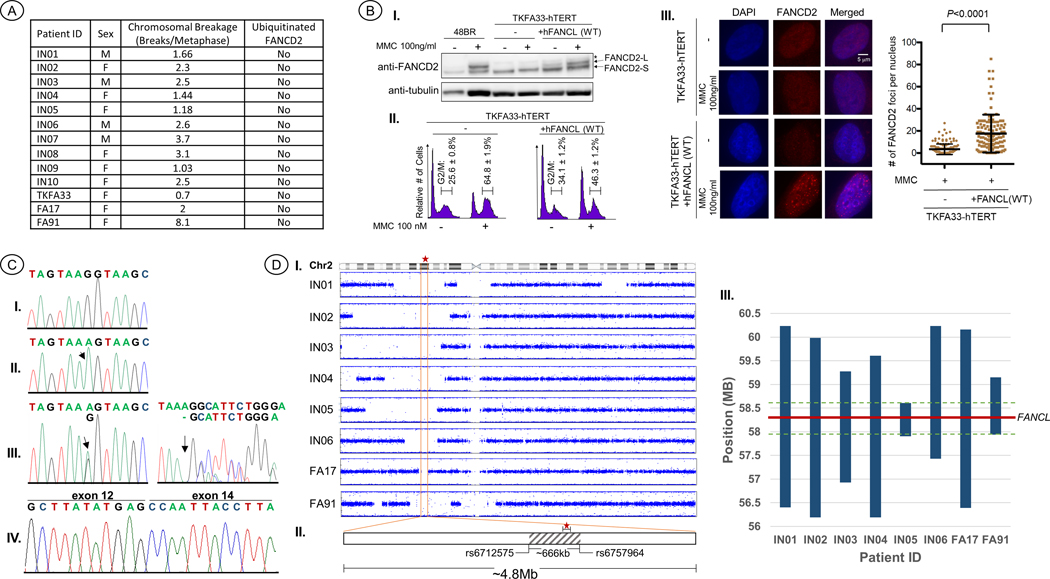
The patients’ crosslink induced chromosomal breakage test results, the standard diagnosis of FA, show a range from 0.7 to 8.1 breaks/metaphase, with a mean of 2 breaks/metaphase (Figure 1A, Supp. Table S1), which is typical of an FA patient (Auerbach, 2009). The age at diagnosis and clinical presentations are presented in Table 1. Patients’ ages at diagnosis ranged from 2.8 to 10 years, with a mean of 5.3 years and median of 5 years. The clinical phenotype of the thirteen patients is generally milder with regard to congenital malformations, with skin pigmentation abnormalities being the most common manifestation, observed in 11/13 (85%) patients. Five (38%) patients exhibited short stature. Skeletal anomalies were also observed in five (38%) patients, including limb and facial malformations and microcephaly. Cardiac, limb, and renal phenotypes were, individually, observed in three patients, but no patients had a diagnosis of VACTERL-H association, as it requires the presentation of at least three of the phenotypes from its name (Alter & Rosenberg, 2013). At the time of diagnosis, BMF was recorded in all 13 patients, with an average age of onset at 5.3 years, and included diagnoses of aplastic anemia, pancytopenia, hypoplastic hypocellular marrow, and thrombocytopenia. Seven patients were born to consanguineous parents, while parental consanguinity was not known for four patients.
Figure 1.
(a) The sex, results from crosslink-induced chromosomal breakage, and FANCD2 ubiquitination are presented for each patient. Diepoxybutane (DEB) was the crosslinking agent used to induce breakage for TKFA33, FA17, and FA91, whereas the rest were treated with mitomycin-C (MMC). (b) Functional evaluation of the FANCL c.1092G>A variant: (I) Western blot analysis of FANCD2 in a representative FANCL patient cell line reveals defective monoubiquitinylation. Lanes 1 and 2 are from an unaffected individual cell line, 48BR. Lanes 3–6 are from TKFA33 patient fibroblast cell line. Lanes 5–6 are transfected with WT FANCL. The asterisk indicates a nonspecific band. Samples in lane 2, 4, and 6 are treated with 100 ng/ml MMC for 24 hr. (II) Cell cycle analysis of FANCL patient cells following treatment with MMC reveals an increase in G2/M phase arrest. Indicated patient cell line with or without MMC treatment and/or WT FANCL transfection were fixed with 70% ethanol, stained with propidium iodide, and analyzed by a FACSCalibur flow cytometer. Percentage of cells (mean and SD) within G2/M phase are indicated. (III) Reduced number of FANCD2 foci upon MMC treatment in FANCL patient cell lines. Indicated cells were fixed and stained with anti-FANCD2 antibody. The left column shows cells stained with DAPI, the middle column shows FANCD2 foci formation, and the right column shows the merged image. In the right panel, each dot represents a single cell with indicated number of FANCD2 foci per nucleus. More than 100 nuclei were scored and shown with mean and standard deviation. The p -value was calculated using unpaired two-tailed Studenťs t test. (c) Electropherograms confirming the FANCL c.1092G>A variant via Sanger sequencing: (I) WT; (II) representative homozygous c.1092G>A alleles observed in IN01-IN09, TKFA33, FA17, and FA91. (III) Compound heterozygous c.1092G>A and c.592delA variants observed in IN10. (IV) cDNA reverse-transcribed from total RNA of a patient homozygous for c.1092G>A, showing skipping of exon 13 in the FANCL transcript. (d) SNP array data: (I) B Allele frequency plots of chromosome 2 showing regions of homozygosity in the genotyped patients. The vertical orange lines indicate the boundaries of the shared region of homozygosity, ~4.8 Mb, and the red star indicates the location of FANCL. (II) The gray patterned area represents the region of shared identity, ~666 kb, among all eight patients, and the rsID of each SNP located at the boundaries. (III) A plot showing the regions of continuous haplotype sharing along chromosome 2. The bars represent the ancestral segment lengths shared by at least two of the patients, the green dotted lines mark the boundaries of the region of shared identity among all eight patients, and the red line indicates the location of FANCL
Table 1.
Clinical observations and hematologic presentation of patients with the FANCL c.1092G>A variant
| Patient ID | FANCL mutations | Ethnic/ Regional origin | Parental Consanguinity | Age at diagnosis | Hematologic phenotypea | Congenital abnormalities | Other clinical observations | VACTERL-He |
|---|---|---|---|---|---|---|---|---|
| IN01* | c.1092G>A; c.1092G>A | Karnataka, India | 3rd degree | 10 | Severe AAb | Hyper- & hypo-pigmentation; bilateral hypoplastic testes | failure to thrive; bronze skin tone | |
| IN02* | c.1092G>A; c.1092G>A | Maharashtra, India | 1st degree | 10 | Pancytopenia | short stature | ||
| IN03* | c.1092G>A; c.1092G>A | Maharashtra, India | 2nd degree | 2.8 | Pancytopenia w/macrocytosis | Café-au lait spots; epistaxis | ||
| IN04* | c.1092G>A; c.1092G>A | Karnataka, India | 2nd degree | 4 | Severe AA | Hyperpigmentation; short stature | ||
| IN05* | c.1092G>A; c.1092G>A | Maharashtra, India | 2nd degree | 5 | Hypoplastic hypocellular marrow | White spots on palm; increased folic acid levels | Increased prothrombin time | |
| IN06* | c.1092G>A; c.1092G>A | Karnataka, India | not known; estimated to be 2nd-degree from genotype data | 3 | Thrombocytopenia | Bilateral index finger; short stature | Brother diagnosed with FA @ age 6 (died of infection/ bleeding) | L |
| IN07 | c.1092G>A; c.1092G>A | Maharashtra, India | 3rd degree | 3 | Pancytopenia | Hyperpigmentation; short stature; triangular facies | ||
| IN08 | c.1092G>A; c.1092G>A | Tamil Nadu, India | not known | 7 | Pancytopenia | Hyperpigmentation; café-au lait spots | ||
| IN09 | c.1092G>A; c.1092G>A | Tamil Nadu, India | not known | 3 | Pancytopenia | Hyperpigmentation; short stature; microcephaly | ||
| IN10 | c.1092G>A; c.592delA | Madhya Pradesh, India | none | 4 | Severe AA | Hyperpigmentation; white spots – face & mouth; deep eyes; high arched palate | Epistaxis | |
| TKFA33 | c.1092G>A; c.1092G>A | Pakistan | not known | 5 | Severe AA (CBTc @ 6 yrs) | Hyperpigmentation; horseshoe kidney | R | |
| FA17* | c.1092G>A; c.1092G>A | Bihar, India | none | 6.5 | AA (BMTd @ 9 yrs) | Café-au lait spots; microcephaly; Patent ductus arteriosus (PDA) | C | |
| FA91* | c.1092G>A; c.1092G>A | Gujarat, India | 2nd degree | 5 | Severe AA (BMT @ 7 yrs) | Café-au lait spots |
SNP array genotyping was performed on DNA from these patients
The cytopenia severity was scored as described (Svahn et al., 2016); BMF status was evaluated by hematologists while referring the samples for the study
AA – aplastic anemia
CBT – cord blood transplantation
BMT – bone marrow transplantation
VACTERL-H: verterbral, anal, cardiac, tracheal-esophageal, renal, limbs, hydrocephaly
Evaluation of FANCD2 ubiquitination informs whether the affected protein is upstream or downstream of the ubiquitination event in the FA DNA repair pathway. In each patient only the non-ubiquitinated form of FANCD2 was present (S-form, 155kDa), indicating a defect in an upstream protein as the source of the disorder (Figure 1A,B).
High-throughput sequencing of the patients’ DNA revealed the FANCL variant, NG_007418.1(NM_018062.3):g.58387243C>T:c.1092G>A, in FA17, FA91, TKFA33, IN05, IN06, IN07, and IN10. All were homozygous (Figure 1C–II) except for IN10 which was compound heterozygous, c.1092G>A; c.592delA (Figure 1C–III). The unusual prevalence of the variant led to the screening of additional FA patients, via Sanger sequencing, who had not been molecularly diagnosed but were known to have an upstream FA gene defect from FANCD2 ubiquitination tests and were also known to not have pathogenic variants in FANCA, FANCC, or FANCG. This screening process led to the discovery of IN01, IN02, IN03, IN04, IN08, and IN09 as homozygous for c.1092G>A.
The c.1092G>A variant is predicted to be synonymous, p.K364=, but is located at the last nucleotide position of exon 13. Three in silico methods produced a consensus prediction that this variant affects splicing with high probability (Supp. Table S2). According to gnomAD (https://gnomad.broadinstitute.org/variant/2-58387243-C-T), this variant (rs577063114) is only observed in 5/250,742 alleles, corresponding to a worldwide frequency of 0.00001994. Interestingly, all five occurrences are heterozygous carriers from South Asia, where the allele frequency is 0.0001634 (5/30,596 alleles). Analysis of cDNA showed that the variant does, indeed, induce aberrant mRNA splicing, c.1021_1092del, skipping exon 13 (Figure 1C–IV) and removing 24 amino acids from the protein product, p.W341_K364del.
To observe the functional defect caused by the c.1021_1092del variant at the protein level, p.W341_K364del, we evaluated the cell line from TKFA33. The cells were transduced with FANCL-FLAG wild-type (WT) lentivirus. In the TKFA33-hTERT cells, the monoubiquitinated long-form of FANCD2 protein was undetectable even after MMC treatment (Figure 1B–I, lanes 3 and 4). However, when transduced with lentivirus encoding FANCL WT, the MMC-induced long form of FANCD2 was restored (Figure 1B–I, lanes 5 and 6). Furthermore, TKFA33 cells showed increased levels of G2/M phase accumulation compared to the complemented cells (Figure 1B–II). Loss of FANCD2 foci formation was also reversed by introduction of WT-FANCL (Figure 1B–III). Collectively, we confirmed that the FANCL c.1021_1092del variant results in a FA cellular phenotype.
We also wanted to evaluate the nuances of the conformational change caused by the p.W341_K364del variation through modelling predictions. FANCL is made up of three domains: the N-terminal or ELF (amino acid residues 1–110), the central-DRDW (amino acid residues 104–294), and the RING domain (amino acid residues 307–365) (Cole, Lewis, & Walden, 2010). The mutant (p.W341_K364del) protein lacks part of the RING domain. Low sequence identity (22%) of FANCL with the resolved structure of the Drosophila melanogaster protein prompted us to, instead, use GenTHREADER, a fold recognition approach, to generate 3D models of the WT and mutant FANCL proteins. The 3D models were validated using PROSA, which revealed that most of the regions were stable wherein the graph value is below zero (Supp. Figure S1A, B). Superimposition of WT with the mutant show better structural superimposition in the N-terminal domain, as expected (Supp. Figure S1C). The deleted 24 residues in the RING domain represent a short helix in anti-parallel beta sheet associated with the UBE2T Complex (Hodson, Purkiss, Miles, & Walden, 2014). This loss of secondary structural element from the RING domain of the mutant results in random coil formation, expected to affect the ubiquitination function of the protein.
DNA from eight patients of Indian origin, IN01, IN02, IN03, IN04, IN05, IN06, FA17, and FA91, along with the parents of IN02, IN03, and FA17, were genotyped using Illumina SNP arrays. We used the genotype data to accomplish three goals: 1) confirm the South Asian ancestral origin of the patients, 2) estimate potential relatedness between individuals, and 3) identify a possible haplotype shared between the patients that could be attributed to an ancestral allele.
To confirm the South Asian ancestral origin of the patients, we performed a multi-dimensional scaling (MDS) analysis using KING (Manichaikul et al., 2010) and included data from 2,501 individuals from the 1000 Genomes Project Phase 3 (Genomes Project et al., 2015) cohort, including 489 individuals from South Asia (SAS). All fourteen genotyped individuals clustered with the SAS population (Supp. Figure S2). We also used KING to infer relatedness between individuals, which confirmed the consanguinity reported for trios IN02 and IN03 and not for FA17 (none reported) in Table 1 and determined there was no evidence of any close relatedness between patients within three degrees, the extent to which the program could reliably predict. Patient IN06, the only genotyped individual whose parental consanguinity was unknown, was estimated to be born to parents with 2nd-degree relatedness by evaluating the genome-wide distribution of autozygosity and obtaining the “FInbred” value using KING.
The genome-wide SNP data corresponded with the reported consanguinity from Table 1, showing extensive autozygosity for the seven patients born to closely related parents. When we evaluated the region on chromosome 2 where FANCL resides, g.58,386,378–58,468,515, we observed a large region of homozygosity in all eight patients, and a shared region of homozygosity extending ~4.8MB (Figure 1D–I), g.55,357,427–60,167,206. Further analysis of this common region of homozygosity revealed a region of shared identity, extending ~666kb and including 160 SNPS, among all eight patients (Figure 1D–II, Supp. Table S3), g.57,941,416–58,607,850.
We estimated the variant’s age, or the time since the most recent common ancestor, using the Gamma method (Gandolfo, Bahlo, & Speed, 2014), which analyzes the contribution from the ancestral chromosome in each individual. We identified the ancestral segment lengths by continuous haplotype sharing between at least two of the patients, with the longest haplotype extending up to ~3.83Mb and the shortest haplotype spanning ~701kb (Figure 1D–III, Supp. Table S3). If we assume an independent genealogy, then the estimated age is equal to 91.6 generations with a 95% CI [55.8 – 151], and if we assume that one generation is equal to 28 years (Moorjani et al., 2016), then the age of the most recent common ancestor is ~2691 years with a 95% CI [1562 – 4228 years].
In summary, we present 13 South Asian FA cases caused by a founder variant in FANCL, a gene that is generally a rare cause of FA worldwide. Although the variant was initially predicted to be synonymous, c.1092G>A;p.K364=, in silico splicing predictors conveyed a high probability to affect splicing, and analysis of cDNA confirmed aberrant splicing that skips exon 13 and removes 24 amino acids, c.1021_c.1092del;p.W341_K364del. The variant is isolated to South Asia, is influenced by a founder effect, and carriers share a common haplotype from an ancestral allele that dates back ~2700 years. As more individuals carrying this variant are discovered, we will be able to better identify the specific sub-populations most at risk. The discovery of population specific disease-causing variants with increased prevalence due to founder effects are important for accurate and efficient genetic screening of FA patients and ensures that we appropriately raise risk awareness and provide proper genetic counseling to FA families.
Supplementary Material
ACKNOWLEDGEMENTS
The study was carried out under the Indo-JSPS program (DST/INT/JSPS/P-206/2015) [BRV and MT], DST/SERB Grant Number EEQ/2016/000510 [BRV], JSPS KAKENHI Grant Number JP15H01738 [MT], and grants from the Ministry of Health, Labor, and Welfare [SK and EI]. We [FXD, RR-B, SCC] acknowledge the support from the intramural research program of the National Human Genome Research Institute, National Institutes of Health. Studies from the IFAR were supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University, Grant # UL1 TR001866, and by RO1HL120922. AS is a HHMI faculty scholar. We thank Adebowale Adeyemo for careful reading of the manuscript and advice on the presentation of genotype data.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Ali AM, Kirby M, Jansen M, Lach FP, Schulte J, Singh TR, . . . Meetei AR (2009). Identification and characterization of mutations in FANCL gene: a second case of Fanconi anemia belonging to FA-L complementation group. Hum Mutat, 30(7), E761–770. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19405097. doi: 10.1002/humu.21032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, & Rosenberg PS (2013). VACTERL-H Association and Fanconi Anemia. Mol Syndromol, 4(1–2), 87–93. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23653579. doi: 10.1159/000346035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane N, Sie D, Dentro S, Ariyurek Y, Kerkhoven L, Joenje H, . . . de Winter JP. (2012). Diagnosis of fanconi anemia: mutation analysis by next-generation sequencing. Anemia, 2012, 132856. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22720145. doi: 10.1155/2012/132856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach AD (2009). Fanconi anemia and its diagnosis. Mutat Res, 668(1–2), 4–10. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19622403. doi: 10.1016/j.mrfmmm.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach AD, Greenbaum J, Pujara K, Batish SD, Bitencourt MA, Kokemohr I, . . . International Fanconi Anemia, R. (2003). Spectrum of sequence variation in the FANCG gene: an International Fanconi Anemia Registry (IFAR) study. Hum Mutat, 21(2), 158–168. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12552564. doi: 10.1002/humu.10166 [DOI] [PubMed] [Google Scholar]
- Butturini A, Gale RP, Verlander PC, Adler-Brecher B, Gillio AP, & Auerbach AD (1994). Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood, 84(5), 1650–1655. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8068955. [PubMed] [Google Scholar]
- Castella M, Pujol R, Callen E, Trujillo JP, Casado JA, Gille H, . . . Surralles J. (2011). Origin, functional role, and clinical impact of Fanconi anemia FANCA mutations. Blood, 117(14), 3759–3769. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21273304. doi: 10.1182/blood-2010-08-299917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharappa SC, Lach FP, Kimble DC, Kamat A, Teer JK, Donovan FX, . . . Program, N. C. S. (2013). Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia. Blood, 121(22), e138–148. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23613520. doi: 10.1182/blood-2012-12-474585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AR, Lewis LP, & Walden H (2010). The structure of the catalytic subunit FANCL of the Fanconi anemia core complex. Nat Struct Mol Biol, 17(3), 294–298. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20154706. doi: 10.1038/nsmb.1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries Y, Lwiwski N, Levitus M, Kuyt B, Israels SJ, Arwert F, . . . Meijers-Heijboer H. (2012). A Dutch Fanconi Anemia FANCC Founder Mutation in Canadian Manitoba Mennonites. Anemia, 2012, 865170. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22701786. doi: 10.1155/2012/865170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Guardiola P, Lewis C, Dokal I, Ebell W, Zatterale A, . . . Mathew CG. (2000). Association of complementation group and mutation type with clinical outcome in fanconi anemia. European Fanconi Anemia Research Group. Blood, 96(13), 4064–4070. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11110674. [PubMed] [Google Scholar]
- Gandolfo LC, Bahlo M, & Speed TP (2014). Dating rare mutations from small samples with dense marker data. Genetics, 197(4), 1315–1327. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24879464. doi: 10.1534/genetics.114.164616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, . . . Abecasis GR. (2015). A global reference for human genetic variation. Nature, 526(7571), 68–74. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26432245. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillio AP, Verlander PC, Batish SD, Giampietro PF, & Auerbach AD (1997). Phenotypic consequences of mutations in the Fanconi anemia FAC gene: an International Fanconi Anemia Registry study. Blood, 90(1), 105–110. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9207444. [PubMed] [Google Scholar]
- Hodson C, Purkiss A, Miles JA, & Walden H (2014). Structure of the human FANCL RING-Ube2T complex reveals determinants of cognate E3-E2 selection. Structure, 22(2), 337–344. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24389026. doi: 10.1016/j.str.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble DC, Lach FP, Gregg SQ, Donovan FX, Flynn EK, Kamat A, . . . Chandrasekharappa SC. (2018). A comprehensive approach to identification of pathogenic FANCA variants in Fanconi anemia patients and their families. Hum Mutat, 39(2), 237–254. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29098742. doi: 10.1002/humu.23366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knies K, Inano S, Ramirez MJ, Ishiai M, Surralles J, Takata M, & Schindler D (2017). Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia. J Clin Invest, 127(8), 3013–3027. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28691929. doi: 10.1172/JCI92069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottemann MC, & Smogorzewska A (2013). Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature, 493(7432), 356–363. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23325218. doi: 10.1038/nature11863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, . . . Auerbach AD. (2003). A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood, 101(4), 1249–1256. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12393516. doi: 10.1182/blood-2002-07-2170 [DOI] [PubMed] [Google Scholar]
- Mamrak NE, Shimamura A, & Howlett NG (2017). Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia. Blood Rev, 31(3), 93–99. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27760710. doi: 10.1016/j.blre.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, & Chen WM (2010). Robust relationship inference in genome-wide association studies. Bioinformatics, 26(22), 2867–2873. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20926424. doi: 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, . . . Wang W. (2003). A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet, 35(2), 165–170. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12973351. doi: 10.1038/ng1241 [DOI] [PubMed] [Google Scholar]
- Moorjani P, Sankararaman S, Fu Q, Przeworski M, Patterson N, & Reich D (2016). A genetic method for dating ancient genomes provides a direct estimate of human generation interval in the last 45,000 years. Proc Natl Acad Sci U S A, 113(20), 5652–5657. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27140627. doi: 10.1073/pnas.1514696113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Hira A, Yoshida K, Muramatsu H, Okuno Y, Shiraishi Y, . . . Takata M (2019). Pathogenic mutations identified by a multimodality approach in 117 Japanese Fanconi anemia patients. Haematologica. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30792206. doi: 10.3324/haematol.2018.207241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling K, Endt D, Hoehn H, & Schindler D (2009). Genotype-phenotype correlations in Fanconi anemia. Mutat Res, 668(1–2), 73–91. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19464302. doi: 10.1016/j.mrfmmm.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Verlander PC, Kaporis A, Liu Q, Zhang Q, Seligsohn U, & Auerbach AD (1995). Carrier frequency of the IVS4 + 4 A-->T mutation of the Fanconi anemia gene FAC in the Ashkenazi Jewish population. Blood, 86(11), 4034–4038. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7492758. [PubMed] [Google Scholar]
- Vetro A, Iascone M, Limongelli I, Ameziane N, Gana S, Della Mina E, . . . Zuffardi O. (2015). Loss-of-Function FANCL Mutations Associate with Severe Fanconi Anemia Overlapping the VACTERL Association. Hum Mutat, 36(5), 562–568. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25754594. doi: 10.1002/humu.22784 [DOI] [PubMed] [Google Scholar]
- Walden H, & Deans AJ (2014). The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys, 43, 257–278. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24773018. doi: 10.1146/annurev-biophys-051013-022737 [DOI] [PubMed] [Google Scholar]
- Wang AT, & Smogorzewska A (2015). SnapShot: Fanconi anemia and associated proteins. Cell, 160(1–2), 354–354 e351. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25594185. doi: 10.1016/j.cell.2014.12.031 [DOI] [PubMed] [Google Scholar]
- Wu W, Liu Y, Zhou Q, Wang Q, Luo F, Xu Z, . . . Xie J (2017). Novel homozygous FANCL mutation and somatic heterozygous SETBP1 mutation in a Chinese girl with Fanconi Anemia. Eur J Med Genet, 60(7), 369–373. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28419882. doi: 10.1016/j.ejmg.2017.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.